当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BCAT-induced autophagy regulates Aβ load through an interdependence of redox state and PKC phosphorylation-implications in Alzheimer's disease.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.freeradbiomed.2020.01.019 M Harris 1 , M El Hindy 1 , M Usmari-Moraes 1 , F Hudd 1 , M Shafei 1 , M Dong 2 , M Hezwani 1 , P Clark 1 , M House 1 , T Forshaw 1 , P Kehoe 3 , M E Conway 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.freeradbiomed.2020.01.019 M Harris 1 , M El Hindy 1 , M Usmari-Moraes 1 , F Hudd 1 , M Shafei 1 , M Dong 2 , M Hezwani 1 , P Clark 1 , M House 1 , T Forshaw 1 , P Kehoe 3 , M E Conway 1
Affiliation
|
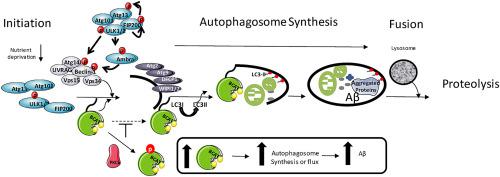
Leucine, nutrient signal and substrate for the branched chain aminotransferase (BCAT) activates the mechanistic target of rapamycin (mTORC1) and regulates autophagic flux, mechanisms implicated in the pathogenesis of neurodegenerative conditions such as Alzheimer's disease (AD). BCAT is upregulated in AD, where a moonlighting role, imparted through its redox-active CXXC motif, has been suggested. Here we demonstrate that the redox state of BCAT signals differential phosphorylation by protein kinase C (PKC) regulating the trafficking of cellular pools of BCAT. We show inter-dependence of BCAT expression and proteins associated with the P13K/Akt/mTORC1 and autophagy signalling pathways. In response to insulin or an increase in ROS, BCATc is trafficked to the membrane and docks via palmitoylation, which is associated with BCATc-induced autophagy through PKC phosphorylation. In response to increased levels of BCATc, as observed in AD, amyloid β (Aβ) levels accumulate due to a shift in autophagic flux. This effect was diminished when incubated with leucine, indicating that dietary levels of amino acids show promise in regulating Aβ load. Together these findings show that increased BCATc expression, reported in human AD brain, will affect autophagy and Aβ load through the interdependence of its redox-regulated phosphorylation offering a novel target to address AD pathology.
中文翻译:

BCAT 诱导的自噬通过氧化还原状态和 PKC 磷酸化在阿尔茨海默病中的相互依赖性来调节 Aβ 负荷。
支链氨基转移酶 (BCAT) 的亮氨酸、营养信号和底物可激活雷帕霉素 (mTORC1) 的机制靶标并调节自噬通量,这些机制与阿尔茨海默病 (AD) 等神经退行性疾病的发病机制有关。BCAT 在 AD 中被上调,有人提出了通过其氧化还原活性 CXXC 基序赋予的兼职角色。在这里,我们证明 BCAT 的氧化还原状态通过调节 BCAT 细胞池的运输的蛋白激酶 C (PKC) 发出差异磷酸化信号。我们展示了与 P13K/Akt/mTORC1 和自噬信号通路相关的 BCAT 表达和蛋白质的相互依赖性。为了响应胰岛素或活性氧的增加,BCATc 通过棕榈酰化被运输到膜上并停靠,这与通过 PKC 磷酸化引起的 BCATc 诱导的自噬有关。正如在 AD 中观察到的那样,为了响应 BCATc 水平的增加,淀粉样蛋白 β (Aβ) 水平由于自噬通量的变化而积累。当与亮氨酸一起孵育时,这种效果会减弱,这表明饮食中的氨基酸水平在调节 Aβ 负荷方面显示出希望。这些发现共同表明,在人类 AD 脑中报告的 BCATc 表达增加将通过其氧化还原调节的磷酸化的相互依赖性影响自噬和 Aβ 负荷,从而为解决 AD 病理学提供新的靶点。表明饮食中的氨基酸水平在调节 Aβ 负荷方面显示出希望。这些发现共同表明,在人类 AD 脑中报告的 BCATc 表达增加将通过其氧化还原调节的磷酸化的相互依赖性影响自噬和 Aβ 负荷,从而为解决 AD 病理学提供新的靶点。表明饮食中的氨基酸水平在调节 Aβ 负荷方面显示出希望。这些发现共同表明,在人类 AD 脑中报告的 BCATc 表达增加将通过其氧化还原调节的磷酸化的相互依赖性影响自噬和 Aβ 负荷,从而为解决 AD 病理学提供新的靶点。
更新日期:2020-01-23
中文翻译:

BCAT 诱导的自噬通过氧化还原状态和 PKC 磷酸化在阿尔茨海默病中的相互依赖性来调节 Aβ 负荷。
支链氨基转移酶 (BCAT) 的亮氨酸、营养信号和底物可激活雷帕霉素 (mTORC1) 的机制靶标并调节自噬通量,这些机制与阿尔茨海默病 (AD) 等神经退行性疾病的发病机制有关。BCAT 在 AD 中被上调,有人提出了通过其氧化还原活性 CXXC 基序赋予的兼职角色。在这里,我们证明 BCAT 的氧化还原状态通过调节 BCAT 细胞池的运输的蛋白激酶 C (PKC) 发出差异磷酸化信号。我们展示了与 P13K/Akt/mTORC1 和自噬信号通路相关的 BCAT 表达和蛋白质的相互依赖性。为了响应胰岛素或活性氧的增加,BCATc 通过棕榈酰化被运输到膜上并停靠,这与通过 PKC 磷酸化引起的 BCATc 诱导的自噬有关。正如在 AD 中观察到的那样,为了响应 BCATc 水平的增加,淀粉样蛋白 β (Aβ) 水平由于自噬通量的变化而积累。当与亮氨酸一起孵育时,这种效果会减弱,这表明饮食中的氨基酸水平在调节 Aβ 负荷方面显示出希望。这些发现共同表明,在人类 AD 脑中报告的 BCATc 表达增加将通过其氧化还原调节的磷酸化的相互依赖性影响自噬和 Aβ 负荷,从而为解决 AD 病理学提供新的靶点。表明饮食中的氨基酸水平在调节 Aβ 负荷方面显示出希望。这些发现共同表明,在人类 AD 脑中报告的 BCATc 表达增加将通过其氧化还原调节的磷酸化的相互依赖性影响自噬和 Aβ 负荷,从而为解决 AD 病理学提供新的靶点。表明饮食中的氨基酸水平在调节 Aβ 负荷方面显示出希望。这些发现共同表明,在人类 AD 脑中报告的 BCATc 表达增加将通过其氧化还原调节的磷酸化的相互依赖性影响自噬和 Aβ 负荷,从而为解决 AD 病理学提供新的靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号