Redox Biology ( IF 10.7 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.redox.2020.101440 Laura Torrente 1 , Nicolas Prieto-Farigua 1 , Aimee Falzone 1 , Cody M Elkins 1 , David A Boothman 2 , Eric B Haura 3 , Gina M DeNicola 1
|
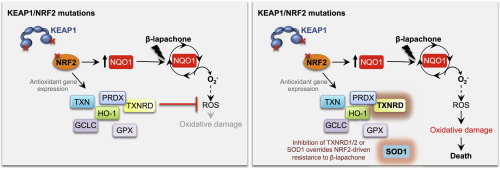
Alterations in the NRF2/KEAP1 pathway result in the constitutive activation of NRF2, leading to the aberrant induction of antioxidant and detoxification enzymes, including NQO1. The NQO1 bioactivatable agent β-lapachone can target cells with high NQO1 expression but relies in the generation of reactive oxygen species (ROS), which are actively scavenged in cells with NRF2/KEAP1 mutations. However, whether NRF2/KEAP1 mutations influence the response to β-lapachone treatment remains unknown. To address this question, we assessed the cytotoxicity of β-lapachone in a panel of NSCLC cell lines bearing either wild-type or mutant KEAP1. We found that, despite overexpression of NQO1, KEAP1 mutant cells were resistant to β-lapachone due to enhanced detoxification of ROS, which prevented DNA damage and cell death. To evaluate whether specific inhibition of the NRF2-regulated antioxidant enzymes could abrogate resistance to β-lapachone, we systematically inhibited the four major antioxidant cellular systems using genetic and/or pharmacologic approaches. We demonstrated that inhibition of the thioredoxin-dependent system or copper-zinc superoxide dismutase (SOD1) could abrogate NRF2-mediated resistance to β-lapachone, while depletion of catalase or glutathione was ineffective. Interestingly, inhibition of SOD1 selectively sensitized KEAP1 mutant cells to β-lapachone exposure. Our results suggest that NRF2/KEAP1 mutational status might serve as a predictive biomarker for response to NQO1-bioactivatable quinones in patients. Further, our results suggest SOD1 inhibition may have potential utility in combination with other ROS inducers in patients with KEAP1/NRF2 mutations.
中文翻译:

抑制 TXNRD 或 SOD1 可克服 NRF2 介导的 β-拉帕酮耐药性。
NRF2/KEAP1 通路的改变会导致 NRF2 的组成型激活,从而导致抗氧化剂和解毒酶(包括 NQO1)的异常诱导。 NQO1 生物激活剂 β-拉帕酮可以靶向 NQO1 高表达的细胞,但依赖于活性氧 (ROS) 的产生,而活性氧在具有 NRF2/KEAP1 突变的细胞中会被主动清除。然而,NRF2/KEAP1 突变是否影响β-拉帕酮治疗的反应仍不清楚。为了解决这个问题,我们评估了 β-拉帕酮在一组带有野生型或突变型 KEAP1 的 NSCLC 细胞系中的细胞毒性。我们发现,尽管 NQO1 过度表达,KEAP1 突变细胞由于 ROS 解毒能力增强而对 β-拉帕酮具有抗性,从而防止了 DNA 损伤和细胞死亡。为了评估特异性抑制 NRF2 调节的抗氧化酶是否可以消除对 β-拉帕酮的耐药性,我们使用遗传和/或药理学方法系统地抑制了四种主要的抗氧化细胞系统。我们证明,抑制硫氧还蛋白依赖性系统或铜锌超氧化物歧化酶(SOD1)可以消除NRF2介导的β-拉帕酮耐药性,而消耗过氧化氢酶或谷胱甘肽则无效。有趣的是,SOD1 的抑制选择性地使 KEAP1 突变细胞对 β-拉帕酮暴露敏感。我们的结果表明,NRF2/KEAP1 突变状态可能作为患者对 NQO1 生物可激活醌反应的预测生物标志物。此外,我们的结果表明,SOD1 抑制可能与其他 ROS 诱导剂联合治疗 KEAP1/NRF2 突变患者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号