Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.jechem.2020.01.021 Xuelei Li , Huilan Guan , Zhijie Ma , Ming Liang , Dawei Song , Hongzhou Zhang , Xixi Shi , Chunliang Li , Lifang Jiao , Lianqi Zhang
|
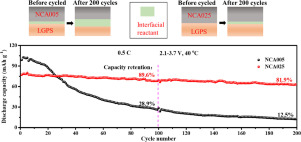
The interfacial instability between Ni-rich layered oxide cathodes and sulfide electrolytes is a serious problem, leading to poor electrochemical properties of all-solid-state lithium batteries (ASSLB). The chemical/electrochemical side reactions are considered to be the origin of the interfacial deterioration. However, the influence of chemical and electrochemical side reactions on the interfacial deterioration is rarely studied specifically. In this work, the deterioration mechanism of the interface between LiNi0.85-xCo0.15AlxO2 and Li10GeP2S12 is investigated in detail by combining in/ex-situ Raman spectra and Electrochemical Impedance Spectroscopy (EIS). It can be determined that chemical side reaction between LiNi0.8Co0.15Al0.05O2 and Li10GeP2S12 will occur immediately once contacted, and the interfacial deterioration becomes more serious after charge-discharge process under the dual effects of chemical and electrochemical side reactions. Moreover, our research reveals that the interfacial stability and the cycle performance of ASSLB can be greatly enhanced by increasing Al-substitution for Ni in LiNi0.85-xCo0.15AlxO2. In particular, the capacity retention of LiNi0.6Co0.15Al0.25O2 cathode after 200 cycles can reach 81.9%, much higher than that of LiNi0.8Co0.15Al0.05O2 cathode (12.5%@200 cycles). This work gives an insight to study the interfacial issues between Ni-rich layered oxide cathode and sulfide electrolyte for ASSLBs.
中文翻译:

原位/异位拉曼光谱结合EIS观察富镍层状氧化物阴极与硫化物电解质之间的界面反应
富镍层状氧化物阴极与硫化物电解质之间的界面不稳定性是一个严重的问题,导致全固态锂电池(ASSLB)的电化学性能较差。化学/电化学副反应被认为是界面劣化的根源。然而,很少专门研究化学和电化学副反应对界面劣化的影响。在这项工作中,LiNi 0.85- x Co 0.15 Al x O 2与Li 10 GeP 2 S 12之间的界面的劣化机理通过结合原位/非原位拉曼光谱和电化学阻抗谱(EIS)进行详细研究。可以确定,LiNi 0.8 Co 0.15 Al 0.05 O 2与Li 10 GeP 2 S 12接触后会立即发生化学副反应,在化学和电化学的双重作用下,充放电后界面劣化更加严重。副反应。此外,我们的研究表明,通过增加LiNi 0.85- x Co 0.15 Al x中的Ni的Al替代可以大大提高ASSLB的界面稳定性和循环性能。O 2。特别地,LiNi 0.6 Co 0.15 Al 0.25 O 2阴极在200次循环后的容量保持率可达到81.9%,远高于LiNi 0.8 Co 0.15 Al 0.05 O 2阴极(12.5%@200次循环)。这项工作为研究ASSLB的富镍层状氧化物阴极和硫化物电解质之间的界面问题提供了见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号