Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible-light-driven selective oxidation of methane to methanol on amorphous FeOOH coupled m-WO3
Fuel ( IF 6.7 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fuel.2020.117104 Juan Yang , Jingyi Hao , Jianping Wei , Jun Dai , Yao Li
Fuel ( IF 6.7 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fuel.2020.117104 Juan Yang , Jingyi Hao , Jianping Wei , Jun Dai , Yao Li
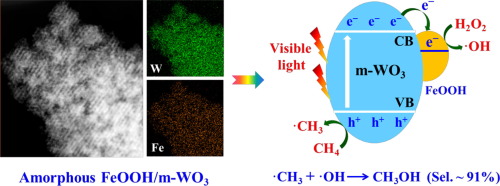
|
Abstract Direct conversion of methane into value-added fuels or chemicals under ambient conditions remains a great challenge. Constructing solar-energy-driven catalytic systems is considered as a promising strategy, but the conversion efficiency and products selectivity are still low, especially for producing alcohol derivatives. Herein, to promote the photocatalytic activity of methane partial oxidation to methanol, a series of FeOOH/m-WO3 consisting of ordered mesoporous WO3 (m-WO3) and highly dispersed amorphous FeOOH were synthesized by using KIT-6 silica as hard template. The prepared FeOOH/m-WO3 catalysts exhibit dramatically improved visible-light catalytic activities toward selective oxidation methane into methanol in the presence of H2O2. A methane conversion rate of 238.6 μmol·g−1·h−1 is achieved over the optimal 1.98% FeOOH/m-WO3, which is 3 times higher than that of pristine m-WO3 (79.2 μmol·g−1·h−1). Moreover, a methanol production rate of 211.2 μmol·g−1·h−1 with a selectivity of 91.0% is obtained on the optimum catalyst under 4 h visible-light irradiation. Significantly, the greatly improved methane conversion and methanol production can be attributed to efficient electron migration from the conduction band of m-WO3 to highly dispersed FeOOH, evidenced by in-situ XPS analysis, transient photocurrent response and photoluminescence spectra. Furthermore, based on radicals trapping experiments and electron spin resonance (ESR) results, methane is primarily activated by photoholes accumulated on the valence band of m-WO3 to generate methyl radicals (·CH3) and the produced hydroxyl radicals (·OH) via decomposing H2O2 by photoelectrons on FeOOH surface are predominant oxidant for methanol generation. Desired methanol is selectively produced via radicals reaction between ·CH3 and ·OH.
中文翻译:

可见光驱动的甲烷在无定形 FeOOH 偶联 m-WO3 上选择性氧化为甲醇
摘要 在环境条件下将甲烷直接转化为增值燃料或化学品仍然是一个巨大的挑战。构建太阳能驱动的催化系统被认为是一种很有前景的策略,但转化效率和产物选择性仍然较低,尤其是在生产醇衍生物方面。在此,为了提高甲烷部分氧化为甲醇的光催化活性,以 KIT-6 二氧化硅为硬模板合成了一系列由有序介孔 WO3 (m-WO3) 和高度分散的无定形 FeOOH 组成的 FeOOH/m-WO3。制备的 FeOOH/m-WO3 催化剂在 H2O2 存在下对甲烷选择性氧化成甲醇表现出显着改善的可见光催化活性。在最佳的 1.98% FeOOH/m-WO3 条件下,甲烷转化率为 238.6 μmol·g-1·h-1,是原始 m-WO3 (79.2 μmol·g-1·h-1) 的 3 倍。此外,在4 h可见光照射下,优化催化剂的甲醇产率为211.2 μmol·g-1·h-1,选择性为91.0%。值得注意的是,原位 XPS 分析、瞬态光电流响应和光致发光光谱证明,甲烷转化率和甲醇产量的大幅提高可归因于从 m-WO3 导带到高度分散的 FeOOH 的有效电子迁移。此外,基于自由基捕获实验和电子自旋共振 (ESR) 结果,甲烷主要由积累在 m-WO3 价带上的光空穴活化产生甲基自由基 (·CH3),而通过 FeOOH 表面上的光电子分解 H2O2 产生的羟基自由基 (·OH) 是生成甲醇的主要氧化剂。通过·CH3 和·OH 之间的自由基反应选择性地产生所需的甲醇。
更新日期:2020-04-01
中文翻译:

可见光驱动的甲烷在无定形 FeOOH 偶联 m-WO3 上选择性氧化为甲醇
摘要 在环境条件下将甲烷直接转化为增值燃料或化学品仍然是一个巨大的挑战。构建太阳能驱动的催化系统被认为是一种很有前景的策略,但转化效率和产物选择性仍然较低,尤其是在生产醇衍生物方面。在此,为了提高甲烷部分氧化为甲醇的光催化活性,以 KIT-6 二氧化硅为硬模板合成了一系列由有序介孔 WO3 (m-WO3) 和高度分散的无定形 FeOOH 组成的 FeOOH/m-WO3。制备的 FeOOH/m-WO3 催化剂在 H2O2 存在下对甲烷选择性氧化成甲醇表现出显着改善的可见光催化活性。在最佳的 1.98% FeOOH/m-WO3 条件下,甲烷转化率为 238.6 μmol·g-1·h-1,是原始 m-WO3 (79.2 μmol·g-1·h-1) 的 3 倍。此外,在4 h可见光照射下,优化催化剂的甲醇产率为211.2 μmol·g-1·h-1,选择性为91.0%。值得注意的是,原位 XPS 分析、瞬态光电流响应和光致发光光谱证明,甲烷转化率和甲醇产量的大幅提高可归因于从 m-WO3 导带到高度分散的 FeOOH 的有效电子迁移。此外,基于自由基捕获实验和电子自旋共振 (ESR) 结果,甲烷主要由积累在 m-WO3 价带上的光空穴活化产生甲基自由基 (·CH3),而通过 FeOOH 表面上的光电子分解 H2O2 产生的羟基自由基 (·OH) 是生成甲醇的主要氧化剂。通过·CH3 和·OH 之间的自由基反应选择性地产生所需的甲醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号