当前位置:
X-MOL 学术
›
Eur. J. Pharm. Biopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An in vitro dissolution-digestion-permeation assay for the study of advanced drug delivery systems.
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.ejpb.2020.01.010 Caroline Alvebratt 1 , Janneke Keemink 1 , Khadijah Edueng 1 , Ocean Cheung 2 , Maria Strømme 2 , Christel A S Bergström 3
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.ejpb.2020.01.010 Caroline Alvebratt 1 , Janneke Keemink 1 , Khadijah Edueng 1 , Ocean Cheung 2 , Maria Strømme 2 , Christel A S Bergström 3
Affiliation
|
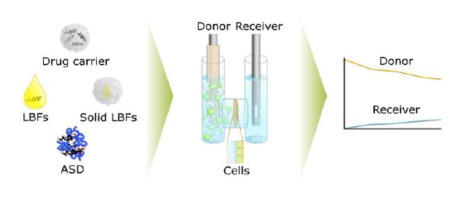
Advanced drug delivery systems (ADDS) are widely explored to overcome poor aqueous solubility of orally administered drugs. However, the prediction of their in vivo performance is challenging, as in vitro models typically do not capture the interplay between processes occurring in the gut. In additions, different models are used to evaluate the different systems. We therefore present a method that allows monitoring of luminal processing (dissolution, digestion) and its interplay with permeation to better inform on the absorption of felodipine formulated as ADDS. Experiments were performed in a µFLUX-apparatus, consisting of two chambers, representing the intestinal and serosal compartment, separated by Caco-2 monolayers. During dissolution-digestion-permeation experiments, ADDS were added to the donor compartment containing simulated intestinal fluid and immobilized lipase. Dissolution and permeation in both compartments were monitored using in situ UV-probes or, when turbidity interfered the measurements, with HPLC analysis. The method showed that all ADDS increased donor and receiver concentrations compared to the condition using crystalline felodipine. A poor correlation between the compartments indicated the need for an serosal compartment to evaluate drug absorption from ADDS. The method enables medium-throughput assessment of: (i) dynamic processes occurring in the small intestine, and (ii) drug concentrations in real-time.
中文翻译:

用于高级药物输送系统研究的体外溶出-消化-渗透测定。
广泛探索了先进的药物输送系统(ADDS),以克服口服药物的不良水溶性。然而,由于体外模型通常无法捕获肠道中发生的过程之间的相互作用,因此对其体内性能的预测具有挑战性。另外,使用不同的模型来评估不同的系统。因此,我们提出了一种方法,该方法可以监测腔的加工(溶解,消化)及其与渗透的相互作用,从而更好地了解配制为ADDS的非洛地平的吸收情况。实验在µFLUX仪器中进行,该仪器由两个腔室组成,分别代表肠腔和浆膜腔,由Caco-2单层分隔。在溶出-消化-渗透实验中,将ADDS添加至包含模拟肠液和固定化脂肪酶的供体隔室。使用原位UV探针或在浊度干扰测量的情况下,通过HPLC分析监测两个隔室中的溶解和渗透。该方法显示,与使用结晶非洛地平的条件相比,所有ADDS均增加了供体和受体的浓度。隔室之间的不良相关性表明需要浆膜隔室来评估药物从ADDS的吸收。该方法可以对以下情况进行中等通量评估:(i)小肠中发生的动态过程,以及(ii)实时进行药物浓度。该方法显示,与使用结晶非洛地平的条件相比,所有ADDS均增加了供体和受体的浓度。隔室之间的不良相关性表明需要浆膜隔室来评估ADDS的药物吸收。该方法可以对以下情况进行中等通量评估:(i)小肠中发生的动态过程,以及(ii)实时进行药物浓度。该方法显示,与使用结晶非洛地平的条件相比,所有ADDS均增加了供体和受体的浓度。隔室之间的不良相关性表明需要浆膜隔室来评估ADDS的药物吸收。该方法可以对以下情况进行中等通量评估:(i)小肠中发生的动态过程,以及(ii)实时药物浓度。
更新日期:2020-01-23
中文翻译:

用于高级药物输送系统研究的体外溶出-消化-渗透测定。
广泛探索了先进的药物输送系统(ADDS),以克服口服药物的不良水溶性。然而,由于体外模型通常无法捕获肠道中发生的过程之间的相互作用,因此对其体内性能的预测具有挑战性。另外,使用不同的模型来评估不同的系统。因此,我们提出了一种方法,该方法可以监测腔的加工(溶解,消化)及其与渗透的相互作用,从而更好地了解配制为ADDS的非洛地平的吸收情况。实验在µFLUX仪器中进行,该仪器由两个腔室组成,分别代表肠腔和浆膜腔,由Caco-2单层分隔。在溶出-消化-渗透实验中,将ADDS添加至包含模拟肠液和固定化脂肪酶的供体隔室。使用原位UV探针或在浊度干扰测量的情况下,通过HPLC分析监测两个隔室中的溶解和渗透。该方法显示,与使用结晶非洛地平的条件相比,所有ADDS均增加了供体和受体的浓度。隔室之间的不良相关性表明需要浆膜隔室来评估药物从ADDS的吸收。该方法可以对以下情况进行中等通量评估:(i)小肠中发生的动态过程,以及(ii)实时进行药物浓度。该方法显示,与使用结晶非洛地平的条件相比,所有ADDS均增加了供体和受体的浓度。隔室之间的不良相关性表明需要浆膜隔室来评估ADDS的药物吸收。该方法可以对以下情况进行中等通量评估:(i)小肠中发生的动态过程,以及(ii)实时进行药物浓度。该方法显示,与使用结晶非洛地平的条件相比,所有ADDS均增加了供体和受体的浓度。隔室之间的不良相关性表明需要浆膜隔室来评估ADDS的药物吸收。该方法可以对以下情况进行中等通量评估:(i)小肠中发生的动态过程,以及(ii)实时药物浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号