Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.cej.2020.124193 Du Chen , Jia Jia , Xue Liao , Lijia Zhou , Zhong-Ting Hu , Bingjun Pan
|
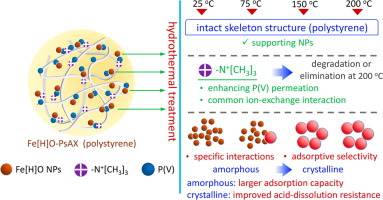
Fe-loaded nanocomposites (NCs) have proven to be a promising category of adsorbents for efficiently removing phosphate [P(V)] from aqueous media. In this study P(V) removal and the possible adsorption mechanisms were investigated for four Fe-loaded NCs, which were developed by loading ferric (hydr)oxide (Fe[H]O) nanoparticles (NPs) of different crystal formations inside polystyrene anion exchanger (PsAX). Fe[H][email protected] (with amorphous Fe[H]O NPs) was synthesized by in situ deposition method under ambient condition, and it served as source material to yield Fe[H][email protected], @150, and @200 by hydrothermal treatment for 24 h under 75, 150, and 200 oC, respectively. Macroscopic experiments revealed that these NCs have decent P(V) adsorption abilities at pH 5-10 except Fe[H][email protected], the functional groups (R-N+[CH3]3) of which severely suffered from degradation or even elimination as evidenced by FT-IR spectra and its exchange capacities. XPS results spectroscopically confirmed three different P(V) adsorption mechanisms, that is, ion-exchange interactions with R-N+[CH3]3, ligand-exchange interactions with amorphous Fe[H]O NPs, and ligand-exchange interactions with crystalline NPs (i.e., hematite). The ion-exchange interactions lack selectivity toward P(V) and would be invalidated by higher concentrations of competing sulfate anions, while ligand-exchange interactions, especially from amorphous Fe[H]O NPs, exhibited great P(V) adsorption selectivity. Acid-dissolution resistance of the loaded Fe[H]O NPs was significantly improved when being transferred into crystal formation, but their P(V) adsorption capacities gradually decreased during transformation process partially because of enlarging particle sizes and thus lessening effective surface areas.
中文翻译:

负载聚苯乙烯阴离子交换剂(PsAX)的Fe纳米复合材料去除磷酸盐:PsAX官能团和三氧化二铁结晶度的影响
负载铁的纳米复合材料(NCs)已被证明是一种有前景的吸附剂,可有效地从水性介质中去除磷酸盐[P(V)]。在这项研究中,研究了通过将聚苯乙烯阴离子内部不同晶体形成的三水合氧化铁(Fe [H] O)纳米粒子(NPs)加载而开发的四个Fe负载的NCs的P(V)去除及其可能的吸附机理。交换器(PsAX)。在环境条件下通过原位沉积法合成了Fe [H] [受电子邮件保护的](具有无定形的Fe [H] O NPs),并作为原料生产了Fe [H] [受电子邮件保护的],@ 150和@ 200在75、150和200 o下进行水热处理24小时C分别。宏观实验表明,这些NC在pH 5-10时具有良好的P(V)吸附能力,但Fe [H] [受电子邮件保护]除外,后者的官能团(RN + [CH 3 ] 3)严重降解甚至消失。 FT-IR光谱及其交换能力证明了这一点。XPS结果在光谱上证实了三种不同的P(V)吸附机理,即与RN + [CH 3 ] 3的离子交换相互作用,与无定形Fe [H] O NP的配体交换相互作用以及与结晶NP(即赤铁矿)的配体交换相互作用。离子交换相互作用对P(V)缺乏选择性,并且会因较高浓度的竞争性硫酸根阴离子而无效,而配体交换相互作用,尤其是无定形Fe [H] O NPs的配体交换相互作用则表现出很大的P(V)吸附选择性。负载的Fe [H] O NPs转移成晶体时,其耐酸溶性得到了显着改善,但它们的P(V)吸附能力在转变过程中逐渐降低,部分原因是增大了粒径,从而减小了有效表面积。











































 京公网安备 11010802027423号
京公网安备 11010802027423号