当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, crystal structure and leishmanicidal activity of new trimethoprim Ru(III), Cu(II) and Pt(II) metal complexes.
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.jinorgbio.2020.111002 Giovani Lindolfo Silva 1 , Júlia Scaff Moreira Dias 1 , Henrique Vieira Reis Silva 1 , Jessica Da Silva Teixeira 2 , Ijaiel Rian Brito De Souza 2 , Elisalva Teixeira Guimarães 3 , Diogo Rodrigo de Magalhães Moreira 4 , Milena Botelho Pereira Soares 2 , Marília Imaculada Frazão Barbosa 1 , Antônio Carlos Doriguetto 1
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.jinorgbio.2020.111002 Giovani Lindolfo Silva 1 , Júlia Scaff Moreira Dias 1 , Henrique Vieira Reis Silva 1 , Jessica Da Silva Teixeira 2 , Ijaiel Rian Brito De Souza 2 , Elisalva Teixeira Guimarães 3 , Diogo Rodrigo de Magalhães Moreira 4 , Milena Botelho Pereira Soares 2 , Marília Imaculada Frazão Barbosa 1 , Antônio Carlos Doriguetto 1
Affiliation
|
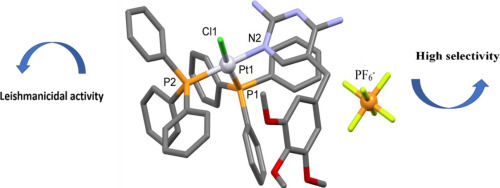
Leishmaniasis is a parasitic disease caused by protozoa of the genus Leishmania, which has very limited treatment options and affects poor and underdeveloped populations. The current treatment is plagued by many complications, such as high toxicity, high cost and resistance to parasites; therefore, novel therapeutic agents are urgently needed. Herein, the synthesis, characterization and in vitro leishmanicidal potential of new complexes with the general formula [RuCl3(TMP)(dppb)] (1), [PtCl(TMP)(PPh3)2]PF6 (2) and [Cu(CH3COO)2(TMP)2]·DMF (3) (dppb = 1,4-bis(diphenylphosphino)butane, PPH3 = triphenylphosphine and TMP = trimethoprim) were evaluated. The complexes were characterized by infrared, UV-vis, cyclic voltammetry, molar conductance measurements, elemental analysis and NMR experiments. Also, the geometry of (2) and (3) were determined by single crystal X-ray diffraction. Despite being less potent against promastigote L. amazonensis proliferation than amphotericin B reference drug (IC50 = 0.09 ± 0.02 μM), complex (2) (IC50 = 3.6 ± 1.5 μM) was several times less cytotoxic (CC50 = 17.8 μM, SI = 4.9) in comparison with amphotericin B (CC50 = 3.3 μM, SI = 36.6) and gentian violet control (CC50 = 0.8 μM). Additionally, complex (2) inhibited J774 macrophage infection and amastigote number by macrophages (IC50 = 6.6 and SI = 2.7). Outstandingly, complex (2) was shown to be a promising candidate for a new leishmanicidal therapeutic agent, considering its biological power combined with low toxicity.
中文翻译:

新型甲氧苄啶Ru(III),Cu(II)和Pt(II)金属配合物的合成,晶体结构和杀螨活性。
利什曼病是由利什曼原虫属的原生动物引起的寄生虫病,其治疗选择非常有限,并且影响贫困和不发达人群。当前的治疗方法受到许多并发症的困扰,例如高毒性,高成本和抗寄生虫性。因此,迫切需要新型治疗剂。本文中,通式为[RuCl3(TMP)(dppb)](1),[PtCl(TMP)(PPh3)2] PF6(2)和[Cu(CH3COO)的新配合物的合成,表征和体外杀菌作用)2(TMP)2]·DMF(3)(dppb = 1,4-双(二苯基膦基)丁烷,PPH3 =三苯基膦,TMP =甲氧苄啶)进行了评价。通过红外,紫外-可见,循环伏安法,摩尔电导测量,元素分析和NMR实验对络合物进行表征。也,通过单晶X射线衍射确定(2)和(3)的几何形状。尽管与两性霉素B参考药物(IC50 = 0.09±0.02μM)相比,对马前体乳杆菌的增殖作用更弱(IC50 = 0.09±0.02μM),复合物(2)(IC50 = 3.6±1.5μM)的细胞毒性却低几倍(CC50 = 17.8μM,SI = 4.9) )与两性霉素B(CC50 = 3.3μM,SI = 36.6)和龙胆紫对照(CC50 = 0.8μM)进行比较。另外,复合物(2)抑制了巨噬细胞的J774巨噬细胞感染和假肢体数目(IC50 = 6.6和SI = 2.7)。出人意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新型的杀菌剂,有望成为一种有前途的候选药物。与两性霉素B参考药物(IC50 = 0.09±0.02μM),复合物(2)(IC50 = 3.6±1.5μM)相比,亚马逊霉素的细胞毒性(CC50 = 17.8μM,SI = 4.9)比两性霉素B(CC50)低几倍= 3.3μM,SI = 36.6)和龙胆紫对照(CC50 = 0.8μM)。另外,复合物(2)抑制了巨噬细胞的J774巨噬细胞感染和假肢体数目(IC50 = 6.6和SI = 2.7)。出乎意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新的杀菌剂,很有希望成为候选药物。与两性霉素B参考药物(IC50 = 0.09±0.02μM),复合物(2)(IC50 = 3.6±1.5μM)相比,亚马逊霉素的细胞毒性(CC50 = 17.8μM,SI = 4.9)比两性霉素B(CC50)低几倍= 3.3μM,SI = 36.6)和龙胆紫对照(CC50 = 0.8μM)。另外,复合物(2)抑制了巨噬细胞的J774巨噬细胞感染和假肢体数目(IC50 = 6.6和SI = 2.7)。出乎意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新的杀菌剂,很有希望成为候选药物。配合物(2)通过巨噬细胞抑制了J774巨噬细胞感染和近鞭毛体数目(IC50 = 6.6和SI = 2.7)。出乎意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新的杀菌剂。配合物(2)通过巨噬细胞抑制了J774巨噬细胞感染和近鞭毛体数目(IC50 = 6.6和SI = 2.7)。出乎意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新的杀菌剂。
更新日期:2020-01-23
中文翻译:

新型甲氧苄啶Ru(III),Cu(II)和Pt(II)金属配合物的合成,晶体结构和杀螨活性。
利什曼病是由利什曼原虫属的原生动物引起的寄生虫病,其治疗选择非常有限,并且影响贫困和不发达人群。当前的治疗方法受到许多并发症的困扰,例如高毒性,高成本和抗寄生虫性。因此,迫切需要新型治疗剂。本文中,通式为[RuCl3(TMP)(dppb)](1),[PtCl(TMP)(PPh3)2] PF6(2)和[Cu(CH3COO)的新配合物的合成,表征和体外杀菌作用)2(TMP)2]·DMF(3)(dppb = 1,4-双(二苯基膦基)丁烷,PPH3 =三苯基膦,TMP =甲氧苄啶)进行了评价。通过红外,紫外-可见,循环伏安法,摩尔电导测量,元素分析和NMR实验对络合物进行表征。也,通过单晶X射线衍射确定(2)和(3)的几何形状。尽管与两性霉素B参考药物(IC50 = 0.09±0.02μM)相比,对马前体乳杆菌的增殖作用更弱(IC50 = 0.09±0.02μM),复合物(2)(IC50 = 3.6±1.5μM)的细胞毒性却低几倍(CC50 = 17.8μM,SI = 4.9) )与两性霉素B(CC50 = 3.3μM,SI = 36.6)和龙胆紫对照(CC50 = 0.8μM)进行比较。另外,复合物(2)抑制了巨噬细胞的J774巨噬细胞感染和假肢体数目(IC50 = 6.6和SI = 2.7)。出人意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新型的杀菌剂,有望成为一种有前途的候选药物。与两性霉素B参考药物(IC50 = 0.09±0.02μM),复合物(2)(IC50 = 3.6±1.5μM)相比,亚马逊霉素的细胞毒性(CC50 = 17.8μM,SI = 4.9)比两性霉素B(CC50)低几倍= 3.3μM,SI = 36.6)和龙胆紫对照(CC50 = 0.8μM)。另外,复合物(2)抑制了巨噬细胞的J774巨噬细胞感染和假肢体数目(IC50 = 6.6和SI = 2.7)。出乎意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新的杀菌剂,很有希望成为候选药物。与两性霉素B参考药物(IC50 = 0.09±0.02μM),复合物(2)(IC50 = 3.6±1.5μM)相比,亚马逊霉素的细胞毒性(CC50 = 17.8μM,SI = 4.9)比两性霉素B(CC50)低几倍= 3.3μM,SI = 36.6)和龙胆紫对照(CC50 = 0.8μM)。另外,复合物(2)抑制了巨噬细胞的J774巨噬细胞感染和假肢体数目(IC50 = 6.6和SI = 2.7)。出乎意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新的杀菌剂,很有希望成为候选药物。配合物(2)通过巨噬细胞抑制了J774巨噬细胞感染和近鞭毛体数目(IC50 = 6.6和SI = 2.7)。出乎意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新的杀菌剂。配合物(2)通过巨噬细胞抑制了J774巨噬细胞感染和近鞭毛体数目(IC50 = 6.6和SI = 2.7)。出乎意料的是,考虑到复合物(2)的生物功能和低毒性,它是一种新的杀菌剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号