当前位置:
X-MOL 学术
›
JAMA Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Objective Response Rate Among Patients With Locally Advanced or Metastatic Sarcoma Treated With Talimogene Laherparepvec in Combination With Pembrolizumab: A Phase 2 Clinical Trial.
JAMA Oncology ( IF 22.5 ) Pub Date : 2020-01-23 , DOI: 10.1001/jamaoncol.2019.6152 Ciara M Kelly 1, 2, 3 , Cristina R Antonescu 4 , Timothy Bowler 4 , Rodrigo Munhoz 4 , Ping Chi 2, 4 , Mark A Dickson 1, 2 , Mrinal M Gounder 1, 2 , Mary Louise Keohan 1, 2 , Sujana Movva 1 , Reena Dholakia 1 , Hamza Ahmad 1 , Matthew Biniakewitz 1 , Mercedes Condy 1 , Haley Phelan 1 , Margaret Callahan 1, 2, 5 , Phillip Wong 1, 5, 6 , Sam Singer 2, 4 , Charlotte Ariyan 2, 4 , Edmund K Bartlett 2, 4 , Aimee Crago 2, 4 , Sam Yoon 2, 4 , Sinchun Hwang 4 , Joseph P Erinjeri 4 , Li-Xuan Qin 4 , William D Tap 1, 2 , Sandra P D'Angelo 1, 2, 5
JAMA Oncology ( IF 22.5 ) Pub Date : 2020-01-23 , DOI: 10.1001/jamaoncol.2019.6152 Ciara M Kelly 1, 2, 3 , Cristina R Antonescu 4 , Timothy Bowler 4 , Rodrigo Munhoz 4 , Ping Chi 2, 4 , Mark A Dickson 1, 2 , Mrinal M Gounder 1, 2 , Mary Louise Keohan 1, 2 , Sujana Movva 1 , Reena Dholakia 1 , Hamza Ahmad 1 , Matthew Biniakewitz 1 , Mercedes Condy 1 , Haley Phelan 1 , Margaret Callahan 1, 2, 5 , Phillip Wong 1, 5, 6 , Sam Singer 2, 4 , Charlotte Ariyan 2, 4 , Edmund K Bartlett 2, 4 , Aimee Crago 2, 4 , Sam Yoon 2, 4 , Sinchun Hwang 4 , Joseph P Erinjeri 4 , Li-Xuan Qin 4 , William D Tap 1, 2 , Sandra P D'Angelo 1, 2, 5
Affiliation
|
Importance
Patients with advanced sarcoma have limited treatment options. Talimogene laherparepvec (T-VEC) has been shown to increase tumor-specific immune activation via augmenting antigen presentation and T-cell priming.
Objective
To examine whether T-VEC in combination with pembrolizumab is associated with increased tumor-infiltrating lymphocyte infiltration and programmed death-ligand 1 expression and thus with increased antitumor activity in patients with locally advanced or metastatic sarcoma.
Design, Setting, and Participants
This open-label, single-institution phase 2 interventional trial of T-VEC plus pembrolizumab enrolled 20 patients with locally advanced or metastatic sarcoma between March 16 and December 4, 2017, for whom at least 1 standard systemic therapy had failed. The median duration of therapy was 16 weeks (range, 7-67 weeks). Reported analyses include data through December 14, 2018.
Intervention
Patients received pembrolizumab (200-mg flat dose) intravenously and T-VEC (first dose, ≤4 mL × 106 plaque-forming units [PFU]/mL; second and subsequent doses, ≤4 mL × 108 PFU/mL) injected into palpable tumor site(s) on day 1 of each 21-day cycle.
Main Outcomes and Measures
The primary end point was objective response rate (ORR; complete response and partial response) at 24 weeks determined by Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1, criteria. Secondary end points included best ORR by immune-related RECIST criteria, progression-free survival rate at 24 weeks, overall survival, and safety.
Results
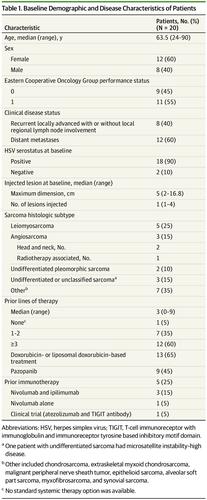
All 20 patients (12 women [60%]; median age, 63.5 years [range, 24-90 years]) were evaluable for response. The study met its primary end point of evaluating the best ORR at 24 weeks determined by RECIST, version 1.1, criteria; the best ORR was 30% (95% CI, 12%-54%; n = 6). The ORR overall was 35% (95% CI, 15%-59%; n = 7). The incidence of grade 3 treatment-related adverse events was low (4 patients [20%]). There were no grade 4 treatment-related adverse events or treatment-related deaths.
Conclusions and Relevance
In this phase 2 clinical trial, treatment with T-VEC plus pembrolizumab was associated with antitumor activity in advanced sarcoma across a range of sarcoma histologic subtypes, with a manageable safety profile. This combination therapy met its predefined primary study end point; further evaluation of T-VEC in combination with pembrolizumab for patients with select sarcoma subtypes is planned.
Trial Registration
ClinicalTrials.gov identifier: NCT03069378.
中文翻译:

Talimogene Laherparepvec 联合 Pembrolizumab 治疗局部晚期或转移性肉瘤患者的客观反应率:2 期临床试验。
重要性 晚期肉瘤患者的治疗选择有限。Talimogene laherparepvec (T-VEC) 已被证明可通过增强抗原呈递和 T 细胞启动来增加肿瘤特异性免疫激活。目的 研究 T-VEC 联合帕博利珠单抗是否与局部晚期或转移性肉瘤患者的肿瘤浸润淋巴细胞浸润增加和程序性死亡配体 1 表达增加相关,从而增加抗肿瘤活性。设计、设置和参与者 2017 年 3 月 16 日至 12 月 4 日期间,T-VEC 加帕博利珠单抗的开放标签、单一机构 2 期介入试验招募了 20 名局部晚期或转移性肉瘤患者,他们至少接受了 1 次标准全身治疗失败了。中位治疗持续时间为 16 周(范围 7-67 周)。报告的分析包括截至 2018 年 12 月 14 日的数据。干预 患者接受静脉内 pembrolizumab(200 mg 固定剂量)和 T-VEC(第一剂,≤4 mL × 106 斑块形成单位 [PFU]/mL;第二剂和后续剂量, ≤4 mL × 108 PFU/mL) 在每个 21 天周期的第 1 天注射到可触及的肿瘤部位。主要成果和措施 主要终点是 24 周时的客观缓解率(ORR;完全缓解和部分缓解),根据实体瘤缓解评估标准 (RECIST) 1.1 版标准确定。次要终点包括免疫相关 RECIST 标准的最佳 ORR、24 周无进展生存率、总生存期和安全性。结果 所有 20 名患者(12 名女性 [60%];中位年龄 63.5 岁 [范围 24-90 岁])均可评估反应。该研究达到了 RECIST 1.1 版标准确定的 24 周时评估最佳 ORR 的主要终点;最佳 ORR 为 30%(95% CI,12%-54%;n = 6)。ORR 总体为 35%(95% CI,15%-59%;n = 7)。3 级治疗相关不良事件的发生率较低(4 名患者 [20%])。没有 4 级治疗相关不良事件或治疗相关死亡。结论和相关性 在这项 2 期临床试验中,T-VEC 加帕博利珠单抗治疗与一系列肉瘤组织学亚型的晚期肉瘤的抗肿瘤活性相关,具有可控的安全性。这种联合疗法达到了预定的主要研究终点;计划进一步评估 T-VEC 与 pembrolizumab 联合治疗特定肉瘤亚型的患者。试验注册临床试验。
更新日期:2020-03-12
中文翻译:

Talimogene Laherparepvec 联合 Pembrolizumab 治疗局部晚期或转移性肉瘤患者的客观反应率:2 期临床试验。
重要性 晚期肉瘤患者的治疗选择有限。Talimogene laherparepvec (T-VEC) 已被证明可通过增强抗原呈递和 T 细胞启动来增加肿瘤特异性免疫激活。目的 研究 T-VEC 联合帕博利珠单抗是否与局部晚期或转移性肉瘤患者的肿瘤浸润淋巴细胞浸润增加和程序性死亡配体 1 表达增加相关,从而增加抗肿瘤活性。设计、设置和参与者 2017 年 3 月 16 日至 12 月 4 日期间,T-VEC 加帕博利珠单抗的开放标签、单一机构 2 期介入试验招募了 20 名局部晚期或转移性肉瘤患者,他们至少接受了 1 次标准全身治疗失败了。中位治疗持续时间为 16 周(范围 7-67 周)。报告的分析包括截至 2018 年 12 月 14 日的数据。干预 患者接受静脉内 pembrolizumab(200 mg 固定剂量)和 T-VEC(第一剂,≤4 mL × 106 斑块形成单位 [PFU]/mL;第二剂和后续剂量, ≤4 mL × 108 PFU/mL) 在每个 21 天周期的第 1 天注射到可触及的肿瘤部位。主要成果和措施 主要终点是 24 周时的客观缓解率(ORR;完全缓解和部分缓解),根据实体瘤缓解评估标准 (RECIST) 1.1 版标准确定。次要终点包括免疫相关 RECIST 标准的最佳 ORR、24 周无进展生存率、总生存期和安全性。结果 所有 20 名患者(12 名女性 [60%];中位年龄 63.5 岁 [范围 24-90 岁])均可评估反应。该研究达到了 RECIST 1.1 版标准确定的 24 周时评估最佳 ORR 的主要终点;最佳 ORR 为 30%(95% CI,12%-54%;n = 6)。ORR 总体为 35%(95% CI,15%-59%;n = 7)。3 级治疗相关不良事件的发生率较低(4 名患者 [20%])。没有 4 级治疗相关不良事件或治疗相关死亡。结论和相关性 在这项 2 期临床试验中,T-VEC 加帕博利珠单抗治疗与一系列肉瘤组织学亚型的晚期肉瘤的抗肿瘤活性相关,具有可控的安全性。这种联合疗法达到了预定的主要研究终点;计划进一步评估 T-VEC 与 pembrolizumab 联合治疗特定肉瘤亚型的患者。试验注册临床试验。











































 京公网安备 11010802027423号
京公网安备 11010802027423号