当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-Assembly of Exendin-4-Derived Dual Peptide Agonists is Mediated by Acylation and Correlated to the Length of Conjugated Fatty Acyl Chains.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-02-05 , DOI: 10.1021/acs.molpharmaceut.9b01195 Martin Wolff 1 , Anja Schüler 1 , Klaus Gast 1 , Robert Seckler 1 , Andreas Evers 2 , Stefania Pfeiffer-Marek 2 , Michael Kurz 2 , Norbert Nagel 2 , Torsten Haack 2 , Michael Wagner 2 , Anja Thalhammer 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-02-05 , DOI: 10.1021/acs.molpharmaceut.9b01195 Martin Wolff 1 , Anja Schüler 1 , Klaus Gast 1 , Robert Seckler 1 , Andreas Evers 2 , Stefania Pfeiffer-Marek 2 , Michael Kurz 2 , Norbert Nagel 2 , Torsten Haack 2 , Michael Wagner 2 , Anja Thalhammer 1
Affiliation
|
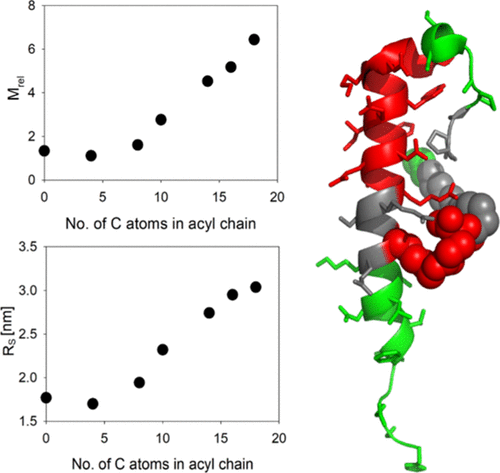
Dual glucagon-like peptide-1/glucagon receptor agonists have emerged as promising candidates for the treatment of diabetes and obesity. Issues of degradation sensitivity and rapid renal clearance are addressed, for example, by the conjugation of peptides to fatty acid chains, promoting reversible albumin binding. We use combined dynamic and static light scattering to directly measure the self-assembly of a set of dual peptide agonists based on the exendin-4 structure with varying fatty acid chain lengths in terms of apparent molecular mass and hydrodynamic radius (RS). We use NMR spectroscopy to gain an insight into the molecular architecture of the assembly. We investigate conformational changes of the monomeric subunits resulting from peptide self-assembly and assembly stability as a function of the fatty acid chain length using circular dichroism and fluorescence spectroscopy. Our results demonstrate that self-assembly of the exendin-4-derived dual agonist peptides is essentially driven by hydrophobic interactions involving the conjugated acyl chains. The fatty acid chain length affects assembly equilibria and the assembly stability, although the peptide subunits in the assembly retain a dynamic secondary structure. The assembly architecture is characterized by juxtaposition of the fatty acyl side chains and a hydrophobic cluster of the peptide moiety. This cluster experiences local conformational changes in the assembly compared to the monomeric unit leading to a reduction in solvent exposure. The N-terminal half of the peptide and a C-terminal loop are not in contact with neighboring peptide subunits in the assemblies. Altogether, our study contributes to a thorough understanding of the association characteristics and the tendency toward self-assembly in response to lipidation. This is important not only to achieve the desired bioavailability but also with respect to the physical stability of peptide solutions.
中文翻译:

Exendin-4衍生的双肽激动剂的自组装介导酰化作用,并与共轭脂肪酰基链的长度相关。
双重胰高血糖素样肽-1 /胰高血糖素受体激动剂已成为治疗糖尿病和肥胖症的有希望的候选者。例如,通过肽与脂肪酸链的缀合,促进可逆白蛋白结合,解决了降解敏感性和快速肾脏清除的问题。我们使用动态和静态光散射组合直接测量基于exendin-4结构的双肽激动剂组的自组装,该结构具有不同的脂肪酸链长(根据表观分子量和流体动力学半径(RS))。我们使用NMR光谱来了解组件的分子结构。我们使用圆二色性和荧光光谱法研究了由于肽的自组装和组装稳定性而导致的单体亚基的构象变化与脂肪酸链长的关系。我们的结果表明,exendin-4衍生的双重激动剂肽的自组装基本上是由涉及共轭酰基链的疏水相互作用驱动的。脂肪酸链长度影响装配平衡和装配稳定性,尽管装配中的肽亚基保留了动态二级结构。组装结构的特征是脂肪酰基侧链与肽部分的疏水簇并置。与单体单元相比,该簇在组装过程中经历局部构象变化,从而减少了溶剂暴露。肽的N端一半和C端环不与装配体中相邻的肽亚基接触。总而言之,我们的研究有助于深入了解缔合特征和对脂质反应的自组装趋势。这不仅对于获得所需的生物利用度很重要,而且对于肽溶液的物理稳定性也很重要。
更新日期:2020-02-06
中文翻译:

Exendin-4衍生的双肽激动剂的自组装介导酰化作用,并与共轭脂肪酰基链的长度相关。
双重胰高血糖素样肽-1 /胰高血糖素受体激动剂已成为治疗糖尿病和肥胖症的有希望的候选者。例如,通过肽与脂肪酸链的缀合,促进可逆白蛋白结合,解决了降解敏感性和快速肾脏清除的问题。我们使用动态和静态光散射组合直接测量基于exendin-4结构的双肽激动剂组的自组装,该结构具有不同的脂肪酸链长(根据表观分子量和流体动力学半径(RS))。我们使用NMR光谱来了解组件的分子结构。我们使用圆二色性和荧光光谱法研究了由于肽的自组装和组装稳定性而导致的单体亚基的构象变化与脂肪酸链长的关系。我们的结果表明,exendin-4衍生的双重激动剂肽的自组装基本上是由涉及共轭酰基链的疏水相互作用驱动的。脂肪酸链长度影响装配平衡和装配稳定性,尽管装配中的肽亚基保留了动态二级结构。组装结构的特征是脂肪酰基侧链与肽部分的疏水簇并置。与单体单元相比,该簇在组装过程中经历局部构象变化,从而减少了溶剂暴露。肽的N端一半和C端环不与装配体中相邻的肽亚基接触。总而言之,我们的研究有助于深入了解缔合特征和对脂质反应的自组装趋势。这不仅对于获得所需的生物利用度很重要,而且对于肽溶液的物理稳定性也很重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号