当前位置:
X-MOL 学术
›
Cell Death Discov.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NF90 stabilizes cyclin E1 mRNA through phosphorylation of NF90-Ser382 by CDK2.
Cell Death Discovery ( IF 6.1 ) Pub Date : 2020-01-22 , DOI: 10.1038/s41420-020-0236-9 Donglin Ding 1, 2, 3 , Huixing Huang 1, 2 , Quanfu Li 1 , Wenbo Yu 2 , Chenji Wang 2 , Haijie Ma 4 , Jiaxue Wu 2 , Yongjun Dang 1 , Long Yu 2 , Wei Jiang 1
Cell Death Discovery ( IF 6.1 ) Pub Date : 2020-01-22 , DOI: 10.1038/s41420-020-0236-9 Donglin Ding 1, 2, 3 , Huixing Huang 1, 2 , Quanfu Li 1 , Wenbo Yu 2 , Chenji Wang 2 , Haijie Ma 4 , Jiaxue Wu 2 , Yongjun Dang 1 , Long Yu 2 , Wei Jiang 1
Affiliation
|
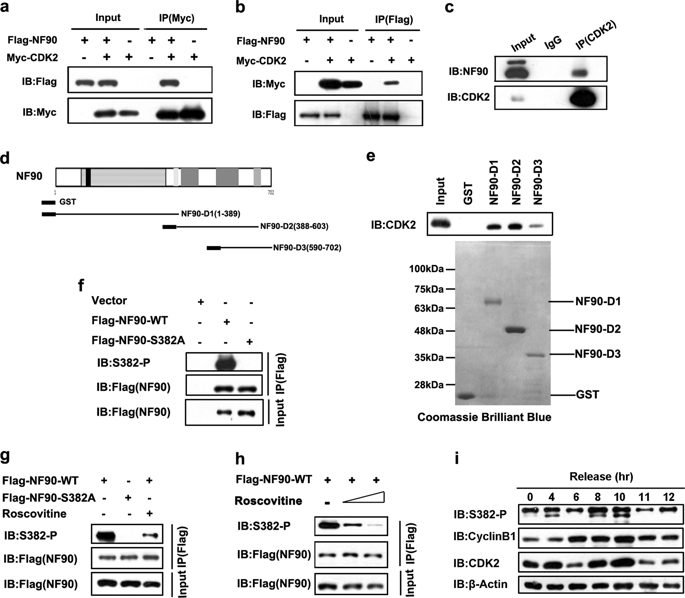
Nuclear factor 90 (NF90), an RNA-binding protein, has been implicated in regulating interleukin-2 (IL-2) and the immune response. It was recently reported that NF90 is upregulated in hepatocellular carcinoma (HCC) tissues and promotes HCC proliferation through upregulating cyclin E1 at the posttranscription level. However, the regulation of NF90 in HCC remains unclear. We demonstrate here that cyclin-dependent kinase (CDK) 2 interacts with NF90 and phosphorylated it at serine382. Mechanistically, phosphorylation of NF90-Ser382 determines the nuclear export of NF90 and stabilization of cyclin E1 mRNA. We also demonstrate that the phosphorylation deficient mutant NF90-S382A inhibits cell growth and induces cell cycle arrest at the G1 phase in HCC cells. Moreover, an NF90-S382A xenograft tumor had a decreased size and weight compared with the wildtype NF90. The NF90-S382A xenograft contained a significantly lower level of the proliferation marker Ki-67. Additionally, in HCC patients, NF90-Ser382 phosphorylation was stronger in tumor than in non-tumor tissues. Clinically, phosphorylation of NF90-Ser382 is significantly associated with larger tumor sizes, higher AFP levels, and shorter overall survival rates. These results suggest NF90-Ser382 phosphorylation serves as a potential diagnosis and prognostic marker and a promising pharmacological target for HCC.
中文翻译:

NF90 通过 CDK2 磷酸化 NF90-Ser382 来稳定细胞周期蛋白 E1 mRNA。
核因子 90 (NF90) 是一种 RNA 结合蛋白,与调节白细胞介素 2 (IL-2) 和免疫反应有关。最近有报道称,NF90在肝细胞癌(HCC)组织中表达上调,并通过在转录后水平上调cyclin E1促进HCC增殖。然而,NF90 在 HCC 中的调控仍不清楚。我们在此证明细胞周期蛋白依赖性激酶 (CDK) 2 与 NF90 相互作用并在丝氨酸 382 处将其磷酸化。从机制上讲,NF90-Ser382 的磷酸化决定了 NF90 的核输出和细胞周期蛋白 E1 mRNA 的稳定性。我们还证明磷酸化缺陷突变体 NF90-S382A 会抑制 HCC 细胞的细胞生长并诱导细胞周期停滞在 G1 期。此外,与野生型NF90相比,NF90-S382A异种移植肿瘤的大小和重量均减小。NF90-S382A 异种移植物含有显着较低水平的增殖标记物 Ki-67。此外,在 HCC 患者中,肿瘤组织中的 NF90-Ser382 磷酸化比非肿瘤组织更强。临床上,NF90-Ser382 磷酸化与较大的肿瘤大小、较高的 AFP 水平和较短的总生存率显着相关。这些结果表明 NF90-Ser382 磷酸化可作为 HCC 的潜在诊断和预后标志物以及有前景的药理学靶点。
更新日期:2020-01-22
中文翻译:

NF90 通过 CDK2 磷酸化 NF90-Ser382 来稳定细胞周期蛋白 E1 mRNA。
核因子 90 (NF90) 是一种 RNA 结合蛋白,与调节白细胞介素 2 (IL-2) 和免疫反应有关。最近有报道称,NF90在肝细胞癌(HCC)组织中表达上调,并通过在转录后水平上调cyclin E1促进HCC增殖。然而,NF90 在 HCC 中的调控仍不清楚。我们在此证明细胞周期蛋白依赖性激酶 (CDK) 2 与 NF90 相互作用并在丝氨酸 382 处将其磷酸化。从机制上讲,NF90-Ser382 的磷酸化决定了 NF90 的核输出和细胞周期蛋白 E1 mRNA 的稳定性。我们还证明磷酸化缺陷突变体 NF90-S382A 会抑制 HCC 细胞的细胞生长并诱导细胞周期停滞在 G1 期。此外,与野生型NF90相比,NF90-S382A异种移植肿瘤的大小和重量均减小。NF90-S382A 异种移植物含有显着较低水平的增殖标记物 Ki-67。此外,在 HCC 患者中,肿瘤组织中的 NF90-Ser382 磷酸化比非肿瘤组织更强。临床上,NF90-Ser382 磷酸化与较大的肿瘤大小、较高的 AFP 水平和较短的总生存率显着相关。这些结果表明 NF90-Ser382 磷酸化可作为 HCC 的潜在诊断和预后标志物以及有前景的药理学靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号