当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemo- and Regioselective Synthesis of Acyl-Cyclohexenes by a Tandem Acceptorless Dehydrogenation-[1,5]-Hydride Shift Cascade
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-22 , DOI: 10.1021/jacs.9b12296 Lewis B Smith 1 , Roly J Armstrong 1 , Daniel Matheau-Raven 1 , Timothy J Donohoe 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-22 , DOI: 10.1021/jacs.9b12296 Lewis B Smith 1 , Roly J Armstrong 1 , Daniel Matheau-Raven 1 , Timothy J Donohoe 1
Affiliation
|
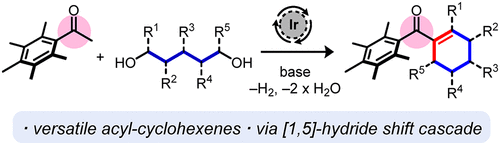
An atom-economical methodology to access substituted acyl-cyclohexenes from pentamethylacetophenone and 1,5-diols is described. This process is catalyzed by an iridium(I) catalyst in conjunction with a bulky electron rich phosphine ligand (CataCXium A) which favors acceptorless dehydrogenation over conjugate reduction to the corresponding cyclohexane. The reaction produces water and hydrogen gas as the sole byproducts and a wide range of functionalized acyl-cyclohexene products can be synthesized using this method in very high yields. A series of control experiments were carried out, which revealed that the process is initiated by acceptorless dehydrogenation of the diol followed by a redox-neutral cascade process, which is independent of the iridium catalyst. Deuterium labeling studies established that the key step of this cascade involves a novel base-mediated [1,5]-hydride shift. The cyclohexenyl ketone products could readily be cleaved under mildly acidic conditions to access a range of valuable substituted cyclohexene derivatives.
中文翻译:

通过串联无受体脱氢-[1,5]-氢化物转移级联反应化学和区域选择性合成酰基环己烯
描述了一种从五甲基苯乙酮和 1,5-二醇中获得取代的酰基环己烯的原子经济方法。该过程由铱 (I) 催化剂与庞大的富电子膦配体 (CataCXium A) 共同催化,该配体有利于无受体脱氢反应,而不是共轭还原成相应的环己烷。该反应产生水和氢气作为唯一的副产物,并且可以使用该方法以非常高的产率合成范围广泛的官能化酰基环己烯产物。进行了一系列对照实验,结果表明该过程是由二醇的无受体脱氢引发的,然后是氧化还原中性级联过程,该过程与铱催化剂无关。氘标记研究表明,该级联的关键步骤涉及一种新型碱介导的 [1,5]-氢化物转移。环己烯基酮产物在弱酸性条件下很容易裂解,以获得一系列有价值的取代环己烯衍生物。
更新日期:2020-01-22
中文翻译:

通过串联无受体脱氢-[1,5]-氢化物转移级联反应化学和区域选择性合成酰基环己烯
描述了一种从五甲基苯乙酮和 1,5-二醇中获得取代的酰基环己烯的原子经济方法。该过程由铱 (I) 催化剂与庞大的富电子膦配体 (CataCXium A) 共同催化,该配体有利于无受体脱氢反应,而不是共轭还原成相应的环己烷。该反应产生水和氢气作为唯一的副产物,并且可以使用该方法以非常高的产率合成范围广泛的官能化酰基环己烯产物。进行了一系列对照实验,结果表明该过程是由二醇的无受体脱氢引发的,然后是氧化还原中性级联过程,该过程与铱催化剂无关。氘标记研究表明,该级联的关键步骤涉及一种新型碱介导的 [1,5]-氢化物转移。环己烯基酮产物在弱酸性条件下很容易裂解,以获得一系列有价值的取代环己烯衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号