当前位置:
X-MOL 学术
›
Cell Death Differ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting LncDACH1 promotes cardiac repair and regeneration after myocardium infarction.
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2020-01-22 , DOI: 10.1038/s41418-020-0492-5 Benzhi Cai 1, 2 , Wenya Ma 1 , Xiuxiu Wang 1 , Natalia Sukhareva 1 , Bingjie Hua 1 , Lai Zhang 1 , Juan Xu 3 , Xingda Li 1 , Shuainan Li 1 , Shenzhen Liu 1 , Meixi Yu 1 , Yan Xu 1 , Ruijie Song 1 , Binbin Xu 1 , Fan Yang 1 , Zhenbo Han 1 , Fengzhi Ding 1 , Qi Huang 1 , Ying Yu 1 , Yue Zhao 1 , Jin Wang 1 , Djibril Bamba 1 , Naufal Zagidullin 4 , Faqian Li 5 , Ye Tian 6 , Zhenwei Pan 1 , Baofeng Yang 1
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2020-01-22 , DOI: 10.1038/s41418-020-0492-5 Benzhi Cai 1, 2 , Wenya Ma 1 , Xiuxiu Wang 1 , Natalia Sukhareva 1 , Bingjie Hua 1 , Lai Zhang 1 , Juan Xu 3 , Xingda Li 1 , Shuainan Li 1 , Shenzhen Liu 1 , Meixi Yu 1 , Yan Xu 1 , Ruijie Song 1 , Binbin Xu 1 , Fan Yang 1 , Zhenbo Han 1 , Fengzhi Ding 1 , Qi Huang 1 , Ying Yu 1 , Yue Zhao 1 , Jin Wang 1 , Djibril Bamba 1 , Naufal Zagidullin 4 , Faqian Li 5 , Ye Tian 6 , Zhenwei Pan 1 , Baofeng Yang 1
Affiliation
|
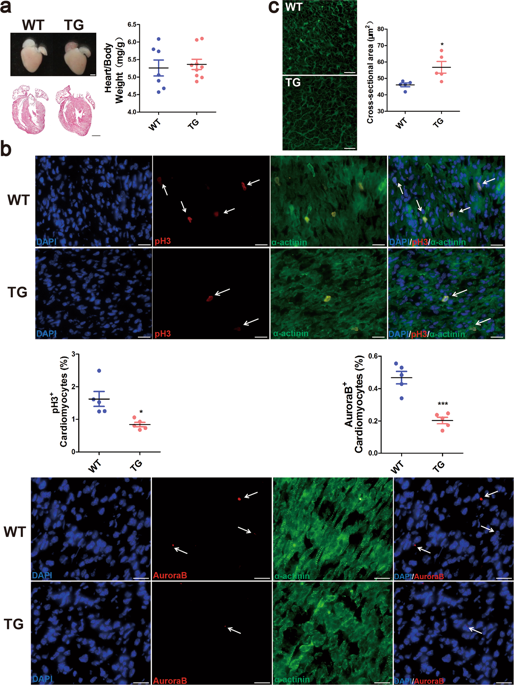
Neonatal mammalian heart maintains a transient regeneration capacity after birth, whereas this regeneration ability gradually loses in the postnatal heart. Thus, the reactivation of cardiomyocyte proliferation is emerging as a key strategy for inducing heart regeneration in adults. We have reported that a highly conserved long noncoding RNA (lncRNA) LncDACH1 was overexpressed in the failing hearts. Here, we found that LncDACH1 was gradually upregulated in the postnatal hearts. Cardiac-specific overexpression of LncDACH1 (TG) in mice suppressed neonatal heart regeneration and worsened cardiac function after apical resection. Conversely, in vivo cardiac conditional knockout of LncDACH1 (CKO) and adenovirus-mediated silencing of endogenous LncDACH1 reactivated cardiomyocyte-proliferative potential and promoted heart regeneration after myocardial infarction (MI) in juvenile and adult mice. Mechanistically, LncDACH1 was found to directly bind to protein phosphatase 1 catalytic subunit alpha (PP1A), and in turn, limit its dephosphorylation activity. Consistently, PP1A siRNA or pharmacological blockers of PP1A abrogated cardiomyocyte mitosis induced by LncDACH1 silencing. Furthermore, LncDACH1 enhanced yes-associated protein 1 (YAP1) phosphorylation and reduced its nuclear translocation by binding PP1A. Verteporfin, a YAP1 inhibitor decreased LncDACH1 silencing-induced cardiomyocyte proliferation. In addition, targeting a conserved fragment of LncDACH1 caused cell cycle re-entry of human iPSC-derived cardiomyocytes. Collectively, LncDACH1 governs heart regeneration in postnatal and ischemic hearts via regulating PP1A/YAP1 signal, which confers a novel therapeutic strategy for ischemic heart diseases.
中文翻译:

靶向 LncDACH1 可促进心肌梗死后的心脏修复和再生。
新生哺乳动物心脏在出生后保持短暂的再生能力,而这种再生能力在出生后的心脏中逐渐丧失。因此,心肌细胞增殖的再激活正在成为诱导成人心脏再生的关键策略。我们已经报道了高度保守的长链非编码 RNA (lncRNA) LncDACH1 在衰竭的心脏中过度表达。在这里,我们发现 LncDACH1 在出生后的心脏中逐渐上调。LncDACH1(TG)在小鼠心脏特异性过表达抑制了新生心脏再生并在心尖切除后使心脏功能恶化。反过来,体内心脏条件敲除 LncDACH1 (CKO) 和腺病毒介导的内源性 LncDACH1 沉默重新激活了心肌细胞增殖潜力,并促进了幼年和成年小鼠心肌梗塞 (MI) 后的心脏再生。从机制上讲,发现 LncDACH1 直接与蛋白磷酸酶 1 催化亚基 α (PP1A) 结合,进而限制其去磷酸化活性。始终如一地,PP1A siRNA 或 PP1A 的药理学阻断剂消除了由 LncDACH1 沉默诱导的心肌细胞有丝分裂。此外,LncDACH1 通过结合 PP1A 增强了 yes 相关蛋白 1 (YAP1) 的磷酸化并减少了其核易位。Verteporfin 是一种 YAP1 抑制剂,可降低 LncDACH1 沉默诱导的心肌细胞增殖。此外,靶向 LncDACH1 的保守片段导致人 iPSC 衍生的心肌细胞重新进入细胞周期。总的来说,LncDACH1 通过调节 PP1A/YAP1 信号来控制出生后和缺血心脏的心脏再生,这为缺血性心脏病提供了一种新的治疗策略。
更新日期:2020-01-22
中文翻译:

靶向 LncDACH1 可促进心肌梗死后的心脏修复和再生。
新生哺乳动物心脏在出生后保持短暂的再生能力,而这种再生能力在出生后的心脏中逐渐丧失。因此,心肌细胞增殖的再激活正在成为诱导成人心脏再生的关键策略。我们已经报道了高度保守的长链非编码 RNA (lncRNA) LncDACH1 在衰竭的心脏中过度表达。在这里,我们发现 LncDACH1 在出生后的心脏中逐渐上调。LncDACH1(TG)在小鼠心脏特异性过表达抑制了新生心脏再生并在心尖切除后使心脏功能恶化。反过来,体内心脏条件敲除 LncDACH1 (CKO) 和腺病毒介导的内源性 LncDACH1 沉默重新激活了心肌细胞增殖潜力,并促进了幼年和成年小鼠心肌梗塞 (MI) 后的心脏再生。从机制上讲,发现 LncDACH1 直接与蛋白磷酸酶 1 催化亚基 α (PP1A) 结合,进而限制其去磷酸化活性。始终如一地,PP1A siRNA 或 PP1A 的药理学阻断剂消除了由 LncDACH1 沉默诱导的心肌细胞有丝分裂。此外,LncDACH1 通过结合 PP1A 增强了 yes 相关蛋白 1 (YAP1) 的磷酸化并减少了其核易位。Verteporfin 是一种 YAP1 抑制剂,可降低 LncDACH1 沉默诱导的心肌细胞增殖。此外,靶向 LncDACH1 的保守片段导致人 iPSC 衍生的心肌细胞重新进入细胞周期。总的来说,LncDACH1 通过调节 PP1A/YAP1 信号来控制出生后和缺血心脏的心脏再生,这为缺血性心脏病提供了一种新的治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号