当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Injectable biodegradable bi-layered capsule for sustained delivery of bevacizumab in treating wet age-related macular degeneration.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.jconrel.2020.01.036 Pengfei Jiang 1 , Francisco J Chaparro 2 , Clayton T Cuddington 1 , Andre F Palmer 1 , Matthew P Ohr 3 , John J Lannutti 2 , Katelyn E Swindle-Reilly 4
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.jconrel.2020.01.036 Pengfei Jiang 1 , Francisco J Chaparro 2 , Clayton T Cuddington 1 , Andre F Palmer 1 , Matthew P Ohr 3 , John J Lannutti 2 , Katelyn E Swindle-Reilly 4
Affiliation
|
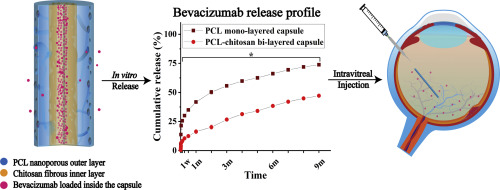
Vascular endothelial growth factor (VEGF) is a key regulator of abnormal blood vessel growth. As such, bevacizumab-based inhibition of VEGF has been the clinically adopted strategy to treat colorectal and breast cancers as well as age-related macular degeneration (AMD). However, as the treatment of vascular diseases often requires a high drug concentration for a long period, the burst release of bevacizumab remains a critical limitation in anti-VEGF-based therapies. Maintaining bevacizumab at high concentrations over extended periods remains challenging due to insufficient drug loading capacity and drug-device interactions. We report the development of a polymeric based bi-layered capsule that could address these challenges by extending the release over one year, thereby providing an effective platform enabling treatment of chronic vascular diseases. Remarkably, the developed capsules have a bi-layered structure which ensures the structural integrity of the injectable capsules and appropriate diffusion of bevacizumab by providing optimal physical trapping and electrostatic interaction. Meanwhile, the central hollow design enables a higher drug loading to meet the need for long-term release of bevacizumab for several months to one year. Using an in vitro drug release assay, we demonstrated that the bi-layered capsule could produce longer-term local drug administration by intravitreal injection compared to previously reported devices. The capsules also present minimal toxicity and maintain anti-VEGF potency, suggesting that our approach may have the potential to treat vascular-related diseases using bevacizumab.
中文翻译:

可注射的可生物降解的双层胶囊,用于持续递送贝伐单抗以治疗与年龄相关的湿性黄斑变性。
血管内皮生长因子(VEGF)是异常血管生长的关键调节剂。因此,基于贝伐单抗的VEGF抑制已成为治疗结直肠癌和乳腺癌以及与年龄相关的黄斑变性(AMD)的临床策略。但是,由于血管疾病的治疗通常需要长期使用高浓度药物,因此贝伐单抗的爆发释放仍然是基于抗VEGF疗法的关键限制。由于不足的载药量和药物与药物的相互作用,维持贝伐单抗长期处于高浓度仍具有挑战性。我们报告了一种基于聚合物的双层胶囊的开发,该胶囊可以通过将释放时间延长一年来应对这些挑战,从而提供了能够治疗慢性血管疾病的有效平台。值得注意的是,已开发的胶囊具有双层结构,可通过提供最佳的物理捕获和静电相互作用来确保可注射胶囊的结构完整性和贝伐单抗的适当扩散。同时,中央空心设计使更高的载药量能够满足贝伐单抗长期释放数月至一年的需要。使用体外药物释放试验,我们证明了与以前报道的装置相比,双层胶囊可以通过玻璃体内注射产生更长期的局部给药。该胶囊还具有最小的毒性,并能维持抗VEGF的功效,
更新日期:2020-01-23
中文翻译:

可注射的可生物降解的双层胶囊,用于持续递送贝伐单抗以治疗与年龄相关的湿性黄斑变性。
血管内皮生长因子(VEGF)是异常血管生长的关键调节剂。因此,基于贝伐单抗的VEGF抑制已成为治疗结直肠癌和乳腺癌以及与年龄相关的黄斑变性(AMD)的临床策略。但是,由于血管疾病的治疗通常需要长期使用高浓度药物,因此贝伐单抗的爆发释放仍然是基于抗VEGF疗法的关键限制。由于不足的载药量和药物与药物的相互作用,维持贝伐单抗长期处于高浓度仍具有挑战性。我们报告了一种基于聚合物的双层胶囊的开发,该胶囊可以通过将释放时间延长一年来应对这些挑战,从而提供了能够治疗慢性血管疾病的有效平台。值得注意的是,已开发的胶囊具有双层结构,可通过提供最佳的物理捕获和静电相互作用来确保可注射胶囊的结构完整性和贝伐单抗的适当扩散。同时,中央空心设计使更高的载药量能够满足贝伐单抗长期释放数月至一年的需要。使用体外药物释放试验,我们证明了与以前报道的装置相比,双层胶囊可以通过玻璃体内注射产生更长期的局部给药。该胶囊还具有最小的毒性,并能维持抗VEGF的功效,










































 京公网安备 11010802027423号
京公网安备 11010802027423号