当前位置:
X-MOL 学术
›
Environ. Pollut.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox reactions between chromium(VI) and hydroquinone: Alternative pathways for polymerization of organic molecules.
Environmental Pollution ( IF 7.6 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.envpol.2020.114024 Yu-Min Tzou , Kai-Yue Chen , Ching-Yun Cheng , Way-Zen Lee , Heng Yi Teah , Yu-Ting Liu
Environmental Pollution ( IF 7.6 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.envpol.2020.114024 Yu-Min Tzou , Kai-Yue Chen , Ching-Yun Cheng , Way-Zen Lee , Heng Yi Teah , Yu-Ting Liu
|
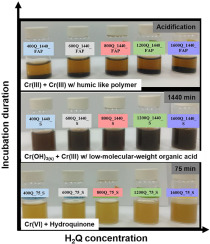
Chromium (VI) reduction by organic compounds is one of the major pathways to alleviate the toxicity and mobility of Cr(VI) in the environment. However, oxidative products of organic molecules receive less scientific concerns. In this study, hydroquinone (H2Q) was used as a representative organic compound to determine the redox reactions with Cr(VI) and the concomitant oxidative products. Spectroscopic analyses showed that Cr(III) hydroxides dominated the precipitates produced during redox reactions of Cr(VI) and H2Q. For the separated filtrates, the acidification induced the oxidative polymerization of organic molecules, accompanied with the complexation with Cr(III). The aromatic domains dominated the chemical structures of the black and fluffy organic polymers, which was different to the natural humic acids due to the shortage of aliphatic chains. Results of linear combination fitting (LCF) for Cr K-edge X-ray absorption near edge structure (XANES) spectra demonstrated that up to 90.4% of Cr inventory in precipitates derived after the acidification of filtrates was Cr(III) complexed with humic-like polymers, suggesting that Cr(III) possibly acted as a linkage among organic molecules during the polymerization processes of H2Q. This study demonstrated that Cr(VI) may lead to the polymerization of organic molecules in an acidic solution, and thus, it could raise scientific awareness that the oxidative decomposition of organic molecules may not be the only pathway while interacting with the strong oxidant of Cr(VI).
中文翻译:

铬(VI)和对苯二酚之间的氧化还原反应:有机分子聚合的替代途径。
有机化合物还原铬(VI)是减轻Cr(VI)在环境中的毒性和迁移性的主要途径之一。但是,有机分子的氧化产物受到的科学关注较少。在这项研究中,对苯二酚(H2Q)被用作代表有机化合物,以确定与Cr(VI)和伴随的氧化产物的氧化还原反应。光谱分析表明,Cr(III)氢氧化物在Cr(VI)和H2Q的氧化还原反应过程中产生的沉淀中占主导地位。对于分离的滤液,酸化诱导有机分子的氧化聚合,并与Cr(III)络合。黑色和蓬松的有机聚合物的化学结构占主导地位,由于缺乏脂肪链,因此与天然腐殖酸不同。Cr K边缘X射线吸收近边缘结构(XANES)光谱的线性组合拟合(LCF)结果表明,滤液酸化后得到的沉淀物中高达90.4%的Cr存量是与腐殖酸络合的Cr(III)。与聚合物类似,表明Cr(III)可能在H2Q的聚合过程中充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。Cr K边缘X射线吸收近边缘结构(XANES)光谱的线性组合拟合(LCF)结果表明,滤液酸化后得到的沉淀物中高达90.4%的Cr存量是与腐殖酸络合的Cr(III)。与聚合物类似,表明Cr(III)可能在H2Q的聚合过程中充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。Cr K边缘X射线吸收近边缘结构(XANES)光谱的线性组合拟合(LCF)结果表明,滤液酸化后得到的沉淀物中高达90.4%的Cr存量是与腐殖酸络合的Cr(III)。与聚合物类似,表明Cr(III)可能在H2Q的聚合过程中充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。这表明Cr(III)在H2Q的聚合过程中可能充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。这表明Cr(III)在H2Q的聚合过程中可能充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。
更新日期:2020-01-23
中文翻译:

铬(VI)和对苯二酚之间的氧化还原反应:有机分子聚合的替代途径。
有机化合物还原铬(VI)是减轻Cr(VI)在环境中的毒性和迁移性的主要途径之一。但是,有机分子的氧化产物受到的科学关注较少。在这项研究中,对苯二酚(H2Q)被用作代表有机化合物,以确定与Cr(VI)和伴随的氧化产物的氧化还原反应。光谱分析表明,Cr(III)氢氧化物在Cr(VI)和H2Q的氧化还原反应过程中产生的沉淀中占主导地位。对于分离的滤液,酸化诱导有机分子的氧化聚合,并与Cr(III)络合。黑色和蓬松的有机聚合物的化学结构占主导地位,由于缺乏脂肪链,因此与天然腐殖酸不同。Cr K边缘X射线吸收近边缘结构(XANES)光谱的线性组合拟合(LCF)结果表明,滤液酸化后得到的沉淀物中高达90.4%的Cr存量是与腐殖酸络合的Cr(III)。与聚合物类似,表明Cr(III)可能在H2Q的聚合过程中充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。Cr K边缘X射线吸收近边缘结构(XANES)光谱的线性组合拟合(LCF)结果表明,滤液酸化后得到的沉淀物中高达90.4%的Cr存量是与腐殖酸络合的Cr(III)。与聚合物类似,表明Cr(III)可能在H2Q的聚合过程中充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。Cr K边缘X射线吸收近边缘结构(XANES)光谱的线性组合拟合(LCF)结果表明,滤液酸化后得到的沉淀物中高达90.4%的Cr存量是与腐殖酸络合的Cr(III)。与聚合物类似,表明Cr(III)可能在H2Q的聚合过程中充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。这表明Cr(III)在H2Q的聚合过程中可能充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。这表明Cr(III)在H2Q的聚合过程中可能充当有机分子之间的键。这项研究表明Cr(VI)可能导致酸性溶液中有机分子聚合,因此,它可以提高科学认识,即有机分子的氧化分解可能不是与Cr的强氧化剂相互作用的唯一途径。 (VI)。







































 京公网安备 11010802027423号
京公网安备 11010802027423号