当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A General and Practical Synthesis of Chiral 1,2‐Oxazetidines
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-01-22 , DOI: 10.1002/ajoc.201900754 Jinggang Yang 1 , Binyu Wu 1 , Lin Hu 1, 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-01-22 , DOI: 10.1002/ajoc.201900754 Jinggang Yang 1 , Binyu Wu 1 , Lin Hu 1, 2
Affiliation
|
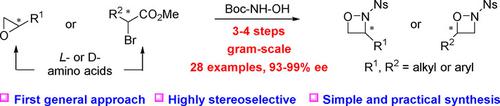
1,2‐Oxazetidines are valuable small strained molecules that could be used to create new and conventionally difficult‐to‐access chemical transformations. Currently, asymmetric methods towards this class of heterocycles are very rare. Herein, we report a general and practical method to access a series of structurally diverse chiral 1,2‐oxazetidines from readily available chiral epoxides and α‐bromo esters in 3–4 steps by using mild Mitsunobu reactions as an efficient ring‐closure approach to form the highly strained four‐membered rings. The new method is operationally simple, and a range of N‐nosyl‐protected 3‐ and 4‐substituted as well as 3,4‐disubstituted chiral 1,2‐oxazetidines could be conveniently prepared in gram‐scale with excellent enantioselectivities (93–99% ee) and good overall yields for the first time.
中文翻译:

通用和实用的手性1,2-氧氮杂环丁烷合成
1,2-氧杂氮杂环丁烷是有价值的小分子小分子,可用于创建新的和传统上难以获得的化学转化。当前,针对此类杂环的非对称方法非常罕见。本文中,我们报告了一种一般实用的方法,通过使用温和的Mitsunobu反应作为有效的闭环方法,以3-4个步骤从易于获得的手性环氧化物和α-溴代酯中获得一系列结构多样的手性1,2-恶唑烷形成高度应变的四元环。该新方法操作简便,可以方便地以克级制备具有良好对映选择性的一系列N炔丙基保护的3和4取代以及3,4-二取代的手性1,2-氧杂环丁烷(93– 99%ee),并首次获得良好的总体收益。
更新日期:2020-01-23
中文翻译:

通用和实用的手性1,2-氧氮杂环丁烷合成
1,2-氧杂氮杂环丁烷是有价值的小分子小分子,可用于创建新的和传统上难以获得的化学转化。当前,针对此类杂环的非对称方法非常罕见。本文中,我们报告了一种一般实用的方法,通过使用温和的Mitsunobu反应作为有效的闭环方法,以3-4个步骤从易于获得的手性环氧化物和α-溴代酯中获得一系列结构多样的手性1,2-恶唑烷形成高度应变的四元环。该新方法操作简便,可以方便地以克级制备具有良好对映选择性的一系列N炔丙基保护的3和4取代以及3,4-二取代的手性1,2-氧杂环丁烷(93– 99%ee),并首次获得良好的总体收益。











































 京公网安备 11010802027423号
京公网安备 11010802027423号