当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Diversity of Amyloid Fibrils and Advances in Their Structure Determination.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-28 , DOI: 10.1021/acs.biochem.9b01069 Dan Li 1, 2 , Cong Liu 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-28 , DOI: 10.1021/acs.biochem.9b01069 Dan Li 1, 2 , Cong Liu 3
Affiliation
|
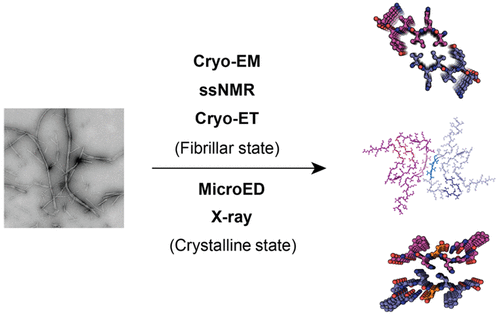
Protein amyloid fibrils are originally identified as pathological entities in a variety of neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease. Recent studies have revealed that amyloid fibrils also serve as functional protein assemblies to fulfill a wide range of biological functions. Deciphering the molecular basis underlying the assembly of amyloid fibrils is essential for understanding their biological and pathological functions. Here, we summarize recent advances in the atomic structure determination of amyloid fibrils formed by both amyloidogenic peptides and full-length proteins. Furthermore, we demonstrate the diversity of amyloid fibrils, with a primary focus on the reversible fibrils, in sequence composition, self-assembled architecture, and physiochemical and pathological properties. Finally, we raise questions that will be answered by the future study of amyloid fibril structure.
中文翻译:

淀粉样原纤维的结构多样性及其结构测定的进展。
蛋白质淀粉样蛋白原纤维最初被确定为各种神经退行性疾病(例如阿尔茨海默氏病和帕金森氏病)的病理实体。最近的研究表明,淀粉样蛋白原纤维还可以作为功能性蛋白质组装体来实现广泛的生物学功能。破译淀粉样蛋白原纤维组装的分子基础对于理解其生物学和病理功能至关重要。在这里,我们总结了由淀粉样蛋白生成肽和全长蛋白形成的淀粉样蛋白原纤维的原子结构测定的最新进展。此外,我们展示了淀粉样蛋白原纤维的多样性,主要侧重于可逆原纤维,序列组成,自组装结构以及理化和病理学性质。最后,
更新日期:2020-01-29
中文翻译:

淀粉样原纤维的结构多样性及其结构测定的进展。
蛋白质淀粉样蛋白原纤维最初被确定为各种神经退行性疾病(例如阿尔茨海默氏病和帕金森氏病)的病理实体。最近的研究表明,淀粉样蛋白原纤维还可以作为功能性蛋白质组装体来实现广泛的生物学功能。破译淀粉样蛋白原纤维组装的分子基础对于理解其生物学和病理功能至关重要。在这里,我们总结了由淀粉样蛋白生成肽和全长蛋白形成的淀粉样蛋白原纤维的原子结构测定的最新进展。此外,我们展示了淀粉样蛋白原纤维的多样性,主要侧重于可逆原纤维,序列组成,自组装结构以及理化和病理学性质。最后,



























 京公网安备 11010802027423号
京公网安备 11010802027423号