当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combining Selective Extraction and Easy Stripping of Lithium Using a Ternary Synergistic Solvent Extraction System through Regulation of Fe3+ Coordination
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-01-22 , DOI: 10.1021/acssuschemeng.9b06432 Hui Su 1, 2, 3 , Zheng Li 4 , Jian Zhang 1, 2, 3 , Wensen Liu 5 , Zhaowu Zhu 1, 2 , Lina Wang 1, 2 , Tao Qi 1, 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-01-22 , DOI: 10.1021/acssuschemeng.9b06432 Hui Su 1, 2, 3 , Zheng Li 4 , Jian Zhang 1, 2, 3 , Wensen Liu 5 , Zhaowu Zhu 1, 2 , Lina Wang 1, 2 , Tao Qi 1, 2
Affiliation
|
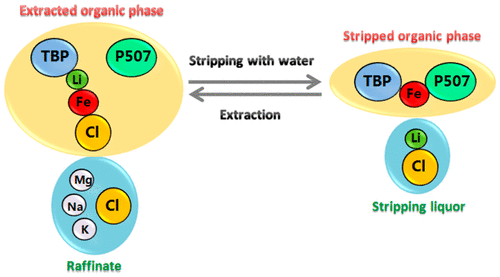
Lithium is becoming increasingly important due to its essential role in lithium-ion batteries. Over 70% of the global lithium resources are found in salt lake brines, where lithium is always accompanied by magnesium. The system of TBP (tributyl phosphate)/FeCl3 has been widely studied for selective lithium extraction from high Mg-containing salt lake brines. However, the system requires a high concentration of HCl for lithium stripping, which causes equipment corrosion, consumes large amounts of reagents, and requires careful neutralization of the organic phase for regeneration. In this study, we develop a novel ternary synergistic solvent extraction system of TBP/FeCl3/P507 (P507 is 2-ethylhexyl phosphonic acid mono 2-ethylhexyl) to significantly enhance the stripping of lithium while largely maintaining lithium extraction capacity. During lithium extraction from Mg-containing solutions, TBP and FeCl3 coordinate with lithium in the form of Li·2TBP·FeCl4, while P507 is not involved in the coordination. When the loaded organic phase is contacted with water, P507 and TBP synergistically coordinate with Fe3+ in the form of FeCl2L·(HL)·2TBP (HL denotes P507), leading to efficient stripping of lithium due to the breaking of the coordination structure of Li·2TBP·FeCl4, and Fe3+ is maintained in the organic phase for the next extraction cycle. The efficient stripping of lithium using only water, instead of high concentration HCl, is a key step forward toward the sustainable extraction of lithium from brines.
中文翻译:

通过调节Fe 3+配位的三元协同溶剂萃取系统,将锂的选择性萃取与易剥离结合起来
锂由于其在锂离子电池中的重要作用而变得越来越重要。在盐湖盐水中发现了全球70%以上的锂资源,而锂总是伴随着镁。已经广泛研究了TBP(磷酸三丁酯)/ FeCl 3的体系,用于从含镁高的盐湖盐水中选择性提取锂。但是,该系统需要高浓度的HCl来汽提锂,这会导致设备腐蚀,消耗大量试剂,并需要小心中和有机相以进行再生。在这项研究中,我们开发了TBP / FeCl 3的新型三元协同溶剂萃取系统/ P507(P507是2-乙基己基膦酸单2-乙基己基)可显着增强锂的汽提作用,同时很大程度上保持锂的萃取能力。在从含Mg的溶液中提取锂时,TBP和FeCl 3以Li·2TBP·FeCl 4的形式与锂配位,而P507不参与配位。当负载的有机相与水接触时,P507和TBP以FeCl 2 L·(HL)·2TBP的形式与Fe 3+协同配位(HL表示P507),导致锂的有效汽提。 Li·2TBP·FeCl 4和Fe 3+的配位结构将其保持在有机相中用于下一个萃取循环。仅用水而不是高浓度HCl进行有效的锂汽提是朝着从盐水中可持续提取锂迈出的关键一步。
更新日期:2020-01-23
中文翻译:

通过调节Fe 3+配位的三元协同溶剂萃取系统,将锂的选择性萃取与易剥离结合起来
锂由于其在锂离子电池中的重要作用而变得越来越重要。在盐湖盐水中发现了全球70%以上的锂资源,而锂总是伴随着镁。已经广泛研究了TBP(磷酸三丁酯)/ FeCl 3的体系,用于从含镁高的盐湖盐水中选择性提取锂。但是,该系统需要高浓度的HCl来汽提锂,这会导致设备腐蚀,消耗大量试剂,并需要小心中和有机相以进行再生。在这项研究中,我们开发了TBP / FeCl 3的新型三元协同溶剂萃取系统/ P507(P507是2-乙基己基膦酸单2-乙基己基)可显着增强锂的汽提作用,同时很大程度上保持锂的萃取能力。在从含Mg的溶液中提取锂时,TBP和FeCl 3以Li·2TBP·FeCl 4的形式与锂配位,而P507不参与配位。当负载的有机相与水接触时,P507和TBP以FeCl 2 L·(HL)·2TBP的形式与Fe 3+协同配位(HL表示P507),导致锂的有效汽提。 Li·2TBP·FeCl 4和Fe 3+的配位结构将其保持在有机相中用于下一个萃取循环。仅用水而不是高浓度HCl进行有效的锂汽提是朝着从盐水中可持续提取锂迈出的关键一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号