当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tuning the Ion Selectivity and Chemical Stability of a Biocellulose Membrane by PFSA Ionomer Reinforcement for Vanadium Redox Flow Battery Applications
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-01-22 , DOI: 10.1021/acssuschemeng.9b06631 Gowthami Palanisamy 1 , T. Sadhasivam 2, 3 , Won-Shik Park 4 , Shin Tae Bae 5 , Sung-Hee Roh 1 , Ho-Young Jung 2, 3
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-01-22 , DOI: 10.1021/acssuschemeng.9b06631 Gowthami Palanisamy 1 , T. Sadhasivam 2, 3 , Won-Shik Park 4 , Shin Tae Bae 5 , Sung-Hee Roh 1 , Ho-Young Jung 2, 3
Affiliation
|
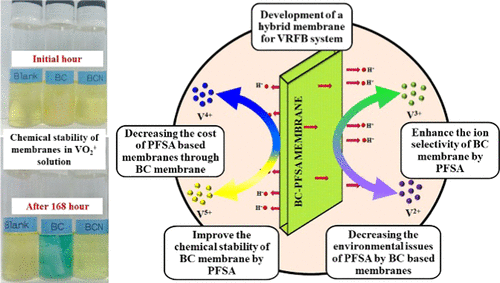
A low-cost biocellulose (BC) membrane is incorporated and coated with the perfluorosulfonic acid (PFSA) ionomer through a new facile preparation route to develop an efficient membrane for vanadium redox flow batteries (VRFBs). Scanning electron microscopy and Brunauer–Emmett–Teller analysis revealed that the BC–PFSA membrane had a dense and smooth surface morphology, thus confirming the efficacious incorporation of PFSA into the pores, between the layers, and on the surface of the microfibrils of the BC membrane. The BC–PFSA membrane exhibited superior physicochemical properties, chemical stability, and electrochemical performance compared to those of the BC membrane. The proton conductivity of the BC–PFSA membrane (0.0605 S/cm) was 12.1 times higher than that of the BC membrane (0.005 S/cm). The presence of proton carriers in the BC–PFSA membrane caused rapid proton mobility through the formation of ionic clusters in the membrane. The pore-filling behavior significantly controlled the vanadium ion crossover in the BC–PFSA membrane. Because of higher selectivity, the charge and discharge behavior of the VRFB system containing the BC–PFSA membrane was superior to that of the BC membrane system. The BC membrane had a low energy efficiency of 63.02% at 80 mA/cm2 in the fifth cycle, while the BC–PFSA membrane showed a much higher energy efficiency of 78.92% under identical conditions. The interaction between the BC microfibrils and the PFSA led to the formation of BC–PFSA, thus increasing the chemical stability of the BC membrane. Furthermore, the BC–PFSA membrane showed a comparable membrane characteristic performance with Nafion and the cost of the BC–PFSA membrane is lower than that of the Nafion membrane. In addition to reducing the cost of the membrane, the BC membrane decreases the environmental impact of PFSA polymer membranes. Because of its remarkable performance, low cost, and great environmental benefits, the BC–PFSA membrane is a promising membrane candidate for VRFB applications.
中文翻译:

通过PFSA离聚物增强钒氧化还原液流电池应用来调节生物纤维素膜的离子选择性和化学稳定性
引入了低成本的生物纤维素(BC)膜,并通过一条新的简便制备路线用全氟磺酸(PFSA)离聚物进行涂覆,从而开发出用于钒氧化还原液流电池(VRFB)的高效膜。扫描电子显微镜和Brunauer-Emmett-Teller分析表明,BC-PFSA膜具有致密而光滑的表面形态,从而证实了PFSA有效地掺入了BC的微孔,各层之间以及其微纤维表面。膜。与BC膜相比,BC-PFSA膜表现出优异的物理化学性能,化学稳定性和电化学性能。BC-PFSA膜的质子传导率(0.0605 S / cm)是BC膜的质子传导率(0.005 S / cm)的12.1倍。BC-PFSA膜中质子载体的存在通过在膜中形成离子簇而引起快速的质子迁移。孔填充行为显着控制了BC-PFSA膜中的钒离子交叉。由于具有更高的选择性,含有BC–PFSA膜的VRFB系统的充电和放电性能优于BC膜系统。BC膜在80 mA / cm时的能效低至63.02%在第五个循环中为2,而BC-PFSA膜在相同条件下显示出更高的能效78.92%。BC微纤维与PFSA之间的相互作用导致BC–PFSA的形成,从而提高了BC膜的化学稳定性。此外,BC-PFSA膜表现出与Nafion相当的膜特性,并且BC-PFSA膜的成本低于Nafion膜。除了降低膜的成本外,BC膜还降低了PFSA聚合物膜对环境的影响。由于其卓越的性能,低成本和巨大的环境效益,BC-PFSA膜是VRFB应用的有前途的膜候选材料。
更新日期:2020-01-23
中文翻译:

通过PFSA离聚物增强钒氧化还原液流电池应用来调节生物纤维素膜的离子选择性和化学稳定性
引入了低成本的生物纤维素(BC)膜,并通过一条新的简便制备路线用全氟磺酸(PFSA)离聚物进行涂覆,从而开发出用于钒氧化还原液流电池(VRFB)的高效膜。扫描电子显微镜和Brunauer-Emmett-Teller分析表明,BC-PFSA膜具有致密而光滑的表面形态,从而证实了PFSA有效地掺入了BC的微孔,各层之间以及其微纤维表面。膜。与BC膜相比,BC-PFSA膜表现出优异的物理化学性能,化学稳定性和电化学性能。BC-PFSA膜的质子传导率(0.0605 S / cm)是BC膜的质子传导率(0.005 S / cm)的12.1倍。BC-PFSA膜中质子载体的存在通过在膜中形成离子簇而引起快速的质子迁移。孔填充行为显着控制了BC-PFSA膜中的钒离子交叉。由于具有更高的选择性,含有BC–PFSA膜的VRFB系统的充电和放电性能优于BC膜系统。BC膜在80 mA / cm时的能效低至63.02%在第五个循环中为2,而BC-PFSA膜在相同条件下显示出更高的能效78.92%。BC微纤维与PFSA之间的相互作用导致BC–PFSA的形成,从而提高了BC膜的化学稳定性。此外,BC-PFSA膜表现出与Nafion相当的膜特性,并且BC-PFSA膜的成本低于Nafion膜。除了降低膜的成本外,BC膜还降低了PFSA聚合物膜对环境的影响。由于其卓越的性能,低成本和巨大的环境效益,BC-PFSA膜是VRFB应用的有前途的膜候选材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号