当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into Trimeric Formation of Nitric Oxide on Cu(111): A Density Functional Theory Study
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jpcc.9b08569 Thanh Ngoc Pham 1, 2 , Yuji Hamamoto 1, 3 , Kouji Inagaki 1, 3 , Do Ngoc Son 2 , Ikutaro Hamada 1, 3 , Yoshitada Morikawa 1, 3, 4
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jpcc.9b08569 Thanh Ngoc Pham 1, 2 , Yuji Hamamoto 1, 3 , Kouji Inagaki 1, 3 , Do Ngoc Son 2 , Ikutaro Hamada 1, 3 , Yoshitada Morikawa 1, 3, 4
Affiliation
|
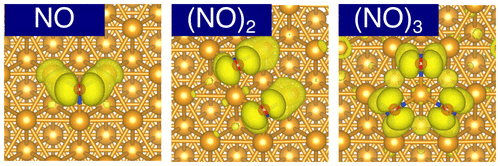
We have studied the adsorption of small nitric oxide (NO) clusters [(NO)n (n = 1–3)] on Cu(111), by means of the van der Waals density functional. We have found that a single NO molecule preferably adsorbs in an upright N-down configuration at the fcc-hollow site, whereas all constituent NO molecules of the NO dimer and trimer adsorb at the fcc-hollow sites, in inclined N-down geometries. Among three NO clusters, the NO trimer is the most stable regardless of lateral periodicities, in good agreement with the scanning tunneling microscopy experiment. van der Waals interaction dominates the stabilization of the NO dimer, while the hybridization among 2π* orbitals of NO plays an important role in addition to the van der Waals interaction in the formation of the NO trimer. The relatively weak interaction between the NO and Cu substrate is crucial for the stabilization of the NO trimer. Our vibrational analysis has revealed that the N–O stretching mode is blue-shifted from monomer to trimer, and the latter agrees well with the electron energy loss spectroscopy data.
中文翻译:

洞察Cu(111)上一氧化氮的三聚体形成:密度泛函理论研究
我们研究了一氧化氮(NO)小簇[(NO)n(n= 1–3)]通过范德华密度函数在Cu(111)上。我们已经发现,单个NO分子优选以直立的N-down构型吸附在fcc-凹陷部位,而NO二聚体和三聚体的所有组成NO分子均以倾斜的N-down几何形状吸附在fcc-凹陷部位。在三个NO簇中,无论横向周期性如何,NO三聚体都是最稳定的,这与扫描隧道显微镜实验非常吻合。范德华相互作用主导着NO二聚体的稳定,而NO的2π*轨道之间的杂化除了在范德瓦尔斯相互作用中形成三聚体外,还起着重要的作用。NO和Cu底物之间相对较弱的相互作用对于NO三聚体的稳定至关重要。
更新日期:2020-01-23
中文翻译:

洞察Cu(111)上一氧化氮的三聚体形成:密度泛函理论研究
我们研究了一氧化氮(NO)小簇[(NO)n(n= 1–3)]通过范德华密度函数在Cu(111)上。我们已经发现,单个NO分子优选以直立的N-down构型吸附在fcc-凹陷部位,而NO二聚体和三聚体的所有组成NO分子均以倾斜的N-down几何形状吸附在fcc-凹陷部位。在三个NO簇中,无论横向周期性如何,NO三聚体都是最稳定的,这与扫描隧道显微镜实验非常吻合。范德华相互作用主导着NO二聚体的稳定,而NO的2π*轨道之间的杂化除了在范德瓦尔斯相互作用中形成三聚体外,还起着重要的作用。NO和Cu底物之间相对较弱的相互作用对于NO三聚体的稳定至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号