Science Bulletin ( IF 18.8 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.scib.2020.01.017 Yildiz Tasdan 1 , Guang-Jian Mei 1 , Yixin Lu 2
|
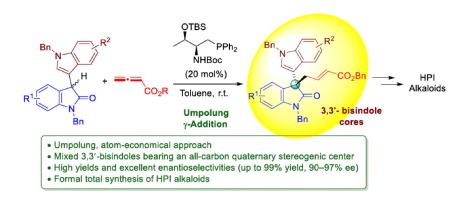
An enantioselective umpolung γ -addition reaction of 3′-indolyl-3-oxindoles to allenoates catalyzed by amino acid-derived bifunctional phosphine catalysts has been developed. A wide range of chiral mixed 3,3′-bisindole scaffolds containing an all-carbon quaternary stereogenic center were obtained in high yields and with excellent enantioselectivities. 3,3′-Bisindoles are valuable synthetic intermediates, the employment of which led to formal total syntheses of (+)-Chimonanthine, (+)-Folicanthine and (-)-Calycanthine, as well as facile creation of useful pyrrolidinoindoline core structure.
中文翻译:

通过膦催化的 umpolung γ-将 3'-吲哚基-3-羟吲哚加成联烯酸酯对映选择性合成混合 3,3'-双吲哚
已经开发了由氨基酸衍生的双功能膦催化剂催化的 3'-吲哚基-3-羟吲哚与联烯酸酯的对映选择性 umpolung γ 加成反应。以高产率和优异的对映选择性获得了多种包含全碳季立体异构中心的手性混合 3,3'-双吲哚支架。3,3'-双吲哚是有价值的合成中间体,使用它可以正式全合成 (+)-Chimonanthine、(+)-Folicanthine 和 (-)-Calycanthine,以及轻松创建有用的吡咯烷二氢吲哚核心结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号