The Lancet Global Health ( IF 19.9 ) Pub Date : 2020-01-22 , DOI: 10.1016/s2214-109x(19)30540-6 Jeremy D Keenan 1 , Ahmed M Arzika 2 , Ramatou Maliki 2 , Sanoussi Elh Adamou 2 , Fatima Ibrahim 2 , Mariama Kiemago 2 , Nana Fatima Galo 2 , Elodie Lebas 3 , Catherine Cook 3 , Benjamin Vanderschelden 3 , Robin L Bailey 4 , Sheila K West 5 , Travis C Porco 6 , Thomas M Lietman 6 ,
|
Background
The Macrolides Oraux pour Réduire les Décès avec un Oeil sur la Résistance (MORDOR) trial found that biannual mass distribution of azithromycin to children younger than 5 years in Niger reduced the primary outcome of all-cause mortality by 18%. We aimed to determine the causes of mortality among deceased children using verbal autopsy.
Methods
In this 2-year cluster-randomised controlled trial, 594 community clusters in Niger were randomly allocated (1:1 ratio) to receive biannual mass distributions of either oral azithromycin (approximately 20 mg per kg of bodyweight) or placebo targeted to children aged 1–59 months. Participants, study investigators, and field workers were masked to treatment allocation. Between Nov 23, 2014, and July 31, 2017, 3615 child deaths were recorded by use of biannual house-to-house censuses, and verbal autopsies were done between May 26, 2015, and May 17, 2018, to identify cause of death. Cause-specific mortality, as assessed by verbal autopsy, was a prespecified secondary outcome. This trial is completed and is registered with ClinicalTrials.gov, NCT02047981.
Findings
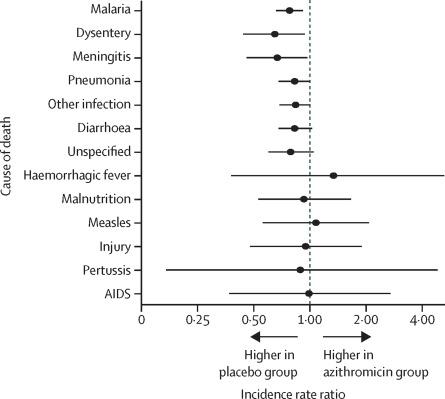
Between Nov 23, 2014, and July 31, 2017, 303 communities (n=40 375 children at baseline) in Niger received mass azithromycin and 291 communities (n=35 747 children at baseline) received placebo. Treatment coverage was 90·3% (SD 10·6) in the azithromycin group and 90·4% (10·1) in the placebo group. No communities were lost to follow-up. In total, 1727 child deaths in the azithromycin group and 1888 child deaths in the placebo group were reported from the population censuses. Of these, the cause of death for 1566 (90·7%) children in the azithromycin group and 1735 (91·9%) children in the placebo group were ascertained by verbal autopsy interviews. In the azithromycin group, 437 (27·9%) deaths were due to malaria, 252 (16·1%) deaths were due to pneumonia, and 234 (14·9%) deaths were due to diarrhoea. In the placebo group, 493 (28·4%) deaths were due to malaria, 275 (15·9%) deaths were due to pneumonia, and 251 (14·5%) deaths were due to diarrhoea. Relative to communities that received placebo, child mortality in communities that received azithromycin was lower for malaria (incidence rate ratio 0·78, 95% CI 0·66–0·92; p=0·0029), dysentery (0·65, 0·44–0·94; p=0·025), meningitis (0·67, 0·46–0·97; p=0·036), and pneumonia (0·83, 0·68–1·00; p=0·051). The distribution of causes of death did not differ significantly between the two study groups (p=0·98).
Interpretation
Mass azithromycin distribution resulted in approximately a third fewer deaths in children aged 1–59 months due to meningitis and dysentery, and a fifth fewer deaths due to malaria and pneumonia. The lack of difference in the distribution of causes of death between the azithromycin and placebo groups could be attributable to the broad spectrum of azithromycin activity and the study setting, in which most childhood deaths were due to infections.
Funding
Bill & Melinda Gates Foundation.
中文翻译:

在尼日尔接受双年度阿奇霉素治疗的社区中,5岁以下儿童的因病死亡率:一项整群随机对照试验的口头尸检结果。
背景
Macrolides Oraux PourRéduirelesDécèsavec un Oil sur laRésistance(MORDOR)试验发现,在尼日尔,每两年一次向5岁以下儿童分发阿奇霉素的质量降低了全因死亡率的主要结局,降低了18%。我们旨在通过口头尸检确定死者的死亡原因。
方法
在这项为期2年的整群随机对照试验中,尼日尔随机分配了594个社区群(比例为1:1)以接受针对1岁儿童的口服阿奇霉素(每公斤体重约20 mg)或安慰剂的半年质量分布–59个月。参与者,研究人员和现场工作人员都被掩盖了治疗分配的方法。在2014年11月23日至2017年7月31日期间,通过每两年一次的住所普查记录了3615例儿童死亡,并在2015年5月26日至2018年5月17日之间进行了口头尸检,以确定死亡原因。通过口头尸检评估的特定原因死亡率是预先确定的次要结局。该试验已完成,并已在ClinicalTrials.gov(NCT02047981)中注册。
发现
在2014年11月23日至2017年7月31日之间,尼日尔的303个社区(基线时n = 40 375名儿童)接受了阿奇霉素的治疗,而291个社区(基线时n = 35 747名儿童)接受了安慰剂。阿奇霉素组的治疗覆盖率为90·3%(SD 10·6),安慰剂组为90·4%(10·1)。没有社区失去后续行动。据人口普查,阿奇霉素组儿童死亡总数为1727,安慰剂组儿童死亡总数为1888。其中,通过语言尸检访谈确定了阿奇霉素组的1566(90·7%)名儿童和安慰剂组的1735(91·9%)儿童的死因。阿奇霉素组中,疟疾导致437例死亡(27·9%),肺炎引起252例死亡(16·1%),腹泻引起234例死亡(14·9%)。在安慰剂组中,疟疾致死493(28·4%),肺炎致死275(15·9%),腹泻致死251(14·5%)。相对于接受安慰剂的社区,接受阿奇霉素的社区的儿童死亡率因疟疾而较低(发生率比0·78,95%CI 0·66-0·92; p = 0·0029),痢疾(0·65, 0·44-0·94; p = 0·025),脑膜炎(0·67、0·46-0·97; p = 0·036)和肺炎(0·83、0·68-1·00) ; p = 0·051)。在两个研究组之间,死亡原因的分布没有显着差异(p = 0·98)。脑膜炎(0·67,0·46-0·97; p = 0·036)和肺炎(0·83,0·68-1·00; p = 0·051)。在两个研究组之间,死亡原因的分布没有显着差异(p = 0·98)。脑膜炎(0·67,0·46-0·97; p = 0·036)和肺炎(0·83,0·68-1·00; p = 0·051)。在两个研究组之间,死亡原因的分布没有显着差异(p = 0·98)。
解释
阿奇霉素的大量分布导致1至59个月大的儿童因脑膜炎和痢疾而死亡的人数减少了约三分之一,而因疟疾和肺炎而死亡的人数则减少了五分之一。阿奇霉素和安慰剂组之间的死亡原因分布缺乏差异可能归因于阿奇霉素活性的广泛范围和研究背景,其中大多数儿童期死亡是由于感染引起的。
资金
比尔和梅琳达·盖茨基金会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号