当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Bioamination of Aromatic Alkanes Using Ammonia: A Multienzymatic Cascade Approach
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-02 , DOI: 10.1002/cctc.201902253 Hui Wang 1 , Yu‐Cong Zheng 1 , Fei‐Fei Chen 1 , Jian‐He Xu 1 , Hui‐Lei Yu 1
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-02 , DOI: 10.1002/cctc.201902253 Hui Wang 1 , Yu‐Cong Zheng 1 , Fei‐Fei Chen 1 , Jian‐He Xu 1 , Hui‐Lei Yu 1
Affiliation
|
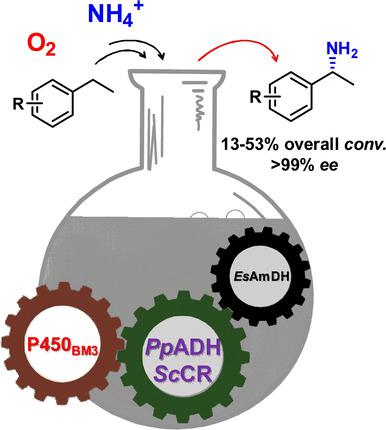
Chiral amines are common drug building blocks and important active pharmaceutical ingredients. Preparing these functionalized compounds from simple materials, such as alkanes, is of great interest. We recently developed an artificial bioamination cascade for the C−H amination of cyclic alkanes by combining P450 monooxygenase, alcohol dehydrogenase, and amine dehydrogenase. Herein, this system has been extended to the synthesis of chiral aromatic amines. In the first hydroxylation step, process optimization increased the conversion to 77 %. Two stereoselectively complementary alcohol dehydrogenases and an amine dehydrogenase were selected for the bioconversion of aromatic hydrocarbons to amines. The amination reaction was optimized with respect to cofactor addition and enzyme dosage. Isopropanol was added to decrease ketone intermediate accumulation in the amination step, which further enhanced the overall conversion. This cascade system converted a panel of hydrocarbon substrates into the corresponding amines with excellent optical purity (>99 % ee) and moderate conversion ratios (13–53 %).
中文翻译:

使用氨对芳烃的对映选择性生物胺化:一种多酶级联方法
手性胺是常见的药物结构单元和重要的活性药物成分。由简单的材料例如烷烃制备这些官能化的化合物是非常令人感兴趣的。我们最近通过结合P450单加氧酶,醇脱氢酶和胺脱氢酶开发了一种用于环烷烃CH胺化的人工生物胺级联反应。本文中,该系统已扩展到手性芳族胺的合成。在第一步羟基化步骤中,工艺优化将转化率提高到77%。选择了两种立体选择性互补的醇脱氢酶和一种胺脱氢酶,用于将芳族烃生物转化为胺。在辅因子添加和酶剂量方面优化了胺化反应。加入异丙醇以减少胺化步骤中酮中间体的积累,这进一步提高了总转化率。该级联系统将一组碳氢化合物底物转化为具有出色的光学纯度(> 99%ee)和中等转换率(13–53%)。
更新日期:2020-04-22
中文翻译:

使用氨对芳烃的对映选择性生物胺化:一种多酶级联方法
手性胺是常见的药物结构单元和重要的活性药物成分。由简单的材料例如烷烃制备这些官能化的化合物是非常令人感兴趣的。我们最近通过结合P450单加氧酶,醇脱氢酶和胺脱氢酶开发了一种用于环烷烃CH胺化的人工生物胺级联反应。本文中,该系统已扩展到手性芳族胺的合成。在第一步羟基化步骤中,工艺优化将转化率提高到77%。选择了两种立体选择性互补的醇脱氢酶和一种胺脱氢酶,用于将芳族烃生物转化为胺。在辅因子添加和酶剂量方面优化了胺化反应。加入异丙醇以减少胺化步骤中酮中间体的积累,这进一步提高了总转化率。该级联系统将一组碳氢化合物底物转化为具有出色的光学纯度(> 99%ee)和中等转换率(13–53%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号