当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrolyte Imbalance Determination of a Vanadium Redox Flow Battery by Potential-Step Analysis of the Initial Charging.
ChemSusChem ( IF 7.5 ) Pub Date : 2020-02-28 , DOI: 10.1002/cssc.201903485 Kirstin Beyer 1 , Jan Grosse Austing 2 , Barbara Satola 1 , Timo Di Nardo 3 , Marco Zobel 1 , Carsten Agert 1
ChemSusChem ( IF 7.5 ) Pub Date : 2020-02-28 , DOI: 10.1002/cssc.201903485 Kirstin Beyer 1 , Jan Grosse Austing 2 , Barbara Satola 1 , Timo Di Nardo 3 , Marco Zobel 1 , Carsten Agert 1
Affiliation
|
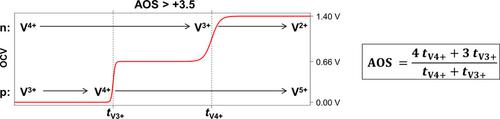
Vanadium redox flow batteries (VRFB) suffer from capacity fades owing to side reactions and crossover effects through the membrane. These processes lead to a deviation of the optimal initial average oxidation state (AOS=+3.5) of vanadium species in both half-cell electrolytes. To rebalance the electrolyte solutions, it is first necessary to determine the current AOS. In this study, a new method was developed that enables an accurate determination of the AOS. A potential-step analysis was performed with mixed electrolyte solutions of both half-cells during the initial charging. The potential was recorded with a simple open-circuit voltage (OCV) cell, and the potential-steps were analyzed. A correlation between the duration of the potential plateaus in the OCV and the amount of vanadium ions of a certain oxidation state in the half-cell electrolytes was found and used to precisely determine the AOS with a maximum error of 3.6 %.
中文翻译:

通过初始充电的电位阶跃分析确定钒氧化还原液流电池的电解质不平衡。
全钒氧化还原液流电池(VRFB)由于副反应和膜的交叉效应而导致容量衰减。这些过程导致两种半电池电解质中钒物质的最佳初始平均氧化态(AOS=+3.5)发生偏差。为了重新平衡电解质溶液,首先需要确定当前的 AOS。在这项研究中,开发了一种能够准确测定 AOS 的新方法。在初始充电期间对两个半电池的混合电解质溶液进行电位阶跃分析。使用简单的开路电压 (OCV) 电池记录电势,并分析电势阶跃。发现了 OCV 中电位平台的持续时间与半电池电解质中特定氧化态的钒离子数量之间的相关性,并用于精确确定 AOS,最大误差为 3.6%。
更新日期:2020-02-28
中文翻译:

通过初始充电的电位阶跃分析确定钒氧化还原液流电池的电解质不平衡。
全钒氧化还原液流电池(VRFB)由于副反应和膜的交叉效应而导致容量衰减。这些过程导致两种半电池电解质中钒物质的最佳初始平均氧化态(AOS=+3.5)发生偏差。为了重新平衡电解质溶液,首先需要确定当前的 AOS。在这项研究中,开发了一种能够准确测定 AOS 的新方法。在初始充电期间对两个半电池的混合电解质溶液进行电位阶跃分析。使用简单的开路电压 (OCV) 电池记录电势,并分析电势阶跃。发现了 OCV 中电位平台的持续时间与半电池电解质中特定氧化态的钒离子数量之间的相关性,并用于精确确定 AOS,最大误差为 3.6%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号