Nature ( IF 50.5 ) Pub Date : 2020-01-22 , DOI: 10.1038/s41586-019-1926-4 Megan Keener 1 , Camden Hunt 1 , Timothy G Carroll 1 , Vladimir Kampel 2 , Roman Dobrovetsky 2 , Trevor W Hayton 1 , Gabriel Ménard 1
|
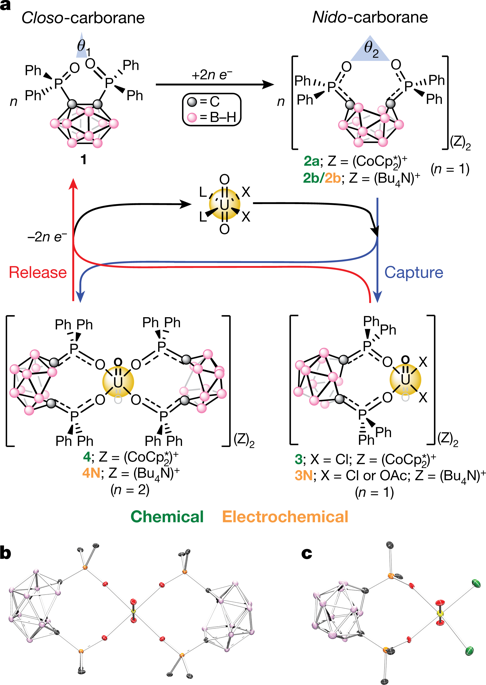
The uranyl ion (UO22+; U(vi) oxidation state) is the most common form of uranium found in terrestrial and aquatic environments and is a central component in nuclear fuel processing and waste remediation efforts. Uranyl capture from either seawater or nuclear waste has been well studied and typically relies on extremely strong chelating/binding affinities to UO22+ using chelating polymers1,2, porous inorganic3,4,5 or carbon-based6,7 materials, as well as homogeneous8 compounds. By contrast, the controlled release of uranyl after capture is less established and can be difficult, expensive or destructive to the initial material2,9. Here we show how harnessing the redox-switchable chelating and donating properties of an ortho-substituted closo-carborane (1,2-(Ph2PO)2-1,2-C2B10H10) cluster molecule can lead to the controlled chemical or electrochemical capture and release of UO22+ in monophasic (organic) or biphasic (organic/aqueous) model solvent systems. This is achieved by taking advantage of the increase in the ligand bite angle when the closo-carborane is reduced to the nido-carborane, resulting in C–C bond rupture and cage opening. The use of electrochemical methods for uranyl capture and release may complement existing sorbent and processing systems.
中文翻译:

用于铀捕获和释放的氧化还原可切换碳硼烷
铀酰离子(UO 2 2+;U( vi ) 氧化态)是在陆地和水生环境中发现的最常见的铀形式,是核燃料加工和废物修复工作的核心成分。从海水或核废料中捕获铀酰已得到充分研究,通常依赖于使用螯合聚合物1,2、多孔无机3,4,5或碳基6,7材料对 UO 2 2+的极强螯合/结合亲和力,以及同质的8种化合物。相比之下,铀酰在捕获后的控制释放不太确定,并且可能很困难、昂贵或对初始材料具有破坏性2,9。在这里,我们展示了如何利用邻位取代的closo -carborane (1,2-(Ph 2 PO) 2 -1,2-C 2 B 10 H 10 ) 簇分子的氧化还原可切换螯合和供体特性来实现UO 2 2+在单相(有机)或双相(有机/水)模型溶剂系统中的受控化学或电化学捕获和释放。这是通过利用在近碳硼烷还原为nido时配体咬合角的增加来实现的-碳硼烷,导致 C-C 键断裂和笼子打开。使用电化学方法捕获和释放铀可以补充现有的吸附剂和处理系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号