Nature ( IF 50.5 ) Pub Date : 2020-01-22 , DOI: 10.1038/s41586-020-1933-5 Haibo Wang 1 , Christian Dienemann 1 , Alexandra Stützer 2 , Henning Urlaub 2, 3 , Alan C M Cheung 4, 5 , Patrick Cramer 1
|
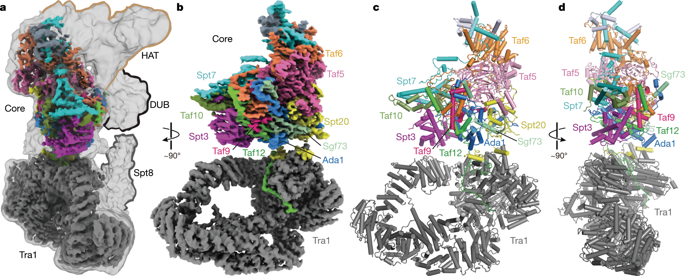
Gene transcription by RNA polymerase II is regulated by activator proteins that recruit the coactivator complexes SAGA (Spt–Ada–Gcn5–acetyltransferase)1,2 and transcription factor IID (TFIID)2,3,4. SAGA is required for all regulated transcription5 and is conserved among eukaryotes6. SAGA contains four modules7,8,9: the activator-binding Tra1 module, the core module, the histone acetyltransferase (HAT) module and the histone deubiquitination (DUB) module. Previous studies provided partial structures10,11,12,13,14, but the structure of the central core module is unknown. Here we present the cryo-electron microscopy structure of SAGA from the yeast Saccharomyces cerevisiae and resolve the core module at 3.3 Å resolution. The core module consists of subunits Taf5, Sgf73 and Spt20, and a histone octamer-like fold. The octamer-like fold comprises the heterodimers Taf6–Taf9, Taf10–Spt7 and Taf12–Ada1, and two histone-fold domains in Spt3. Spt3 and the adjacent subunit Spt8 interact with the TATA box-binding protein (TBP)2,7,15,16,17. The octamer-like fold and its TBP-interacting region are similar in TFIID, whereas Taf5 and the Taf6 HEAT domain adopt distinct conformations. Taf12 and Spt20 form flexible connections to the Tra1 module, whereas Sgf73 tethers the DUB module. Binding of a nucleosome to SAGA displaces the HAT and DUB modules from the core-module surface, allowing the DUB module to bind one face of an ubiquitinated nucleosome.
中文翻译:

转录辅激活子SAGA的结构
RNA 聚合酶 II 的基因转录受到激活蛋白的调节,激活蛋白招募共激活复合物 SAGA(Spt-Ada-Gcn5-乙酰转移酶) 1,2和转录因子 IID (TFIID) 2,3,4 。 SAGA 是所有调控转录所必需的5 ,并且在真核生物中是保守的6 。 SAGA包含四个模块7、8、9 :激活剂结合Tra1模块、核心模块、组蛋白乙酰转移酶(HAT)模块和组蛋白去泛素化(DUB)模块。先前的研究提供了部分结构10,11,12,13,14 ,但中央核心模块的结构未知。在这里,我们展示了来自酿酒酵母的 SAGA 的冷冻电子显微镜结构,并以 3.3 Å 的分辨率解析了核心模块。核心模块由 Taf5、Sgf73 和 Spt20 亚基以及组蛋白八聚体样折叠组成。八聚体样折叠包括异二聚体 Taf6-Taf9、Taf10-Spt7 和 Taf12-Ada1,以及 Spt3 中的两个组蛋白折叠结构域。 Spt3 和相邻的 Spt8 亚基与 TATA 盒结合蛋白 (TBP) 相互作用2,7,15,16,17 。 TFIID 中的八聚体样折叠及其 TBP 相互作用区域相似,而 Taf5 和 Taf6 HEAT 结构域采用不同的构象。 Taf12 和 Spt20 形成与 Tra1 模块的灵活连接,而 Sgf73 则连接 DUB 模块。核小体与 SAGA 的结合将 HAT 和 DUB 模块从核心模块表面取代,从而允许 DUB 模块结合泛素化核小体的一个面。










































 京公网安备 11010802027423号
京公网安备 11010802027423号