Nature ( IF 50.5 ) Pub Date : 2020-01-22 , DOI: 10.1038/s41586-020-1936-2 Clare Rollie 1 , Anne Chevallereau 1 , Bridget N J Watson 1 , Te-Yuan Chyou 2 , Olivier Fradet 1 , Isobel McLeod 1 , Peter C Fineran 3, 4 , Chris M Brown 2, 4 , Sylvain Gandon 5 , Edze R Westra 1
|
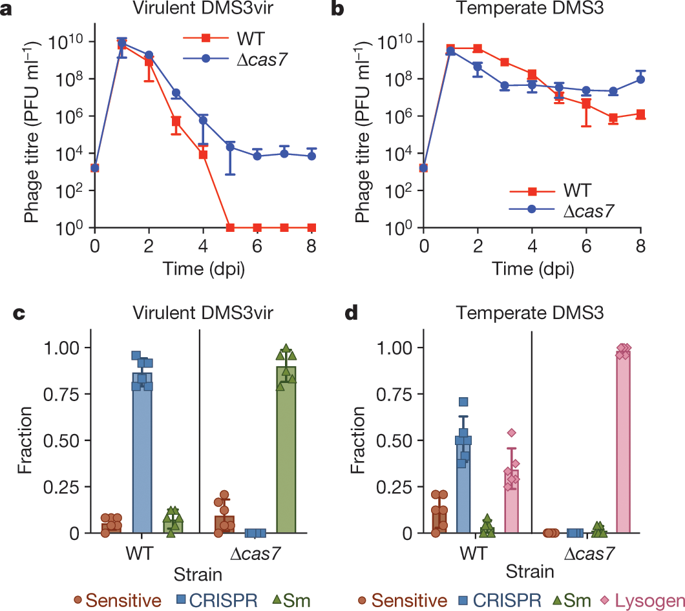
On infection of their host, temperate viruses that infect bacteria (bacteriophages; hereafter referred to as phages) enter either a lytic or a lysogenic cycle. The former results in lysis of bacterial cells and phage release (resulting in horizontal transmission), whereas lysogeny is characterized by the integration of the phage into the host genome, and dormancy (resulting in vertical transmission)1. Previous co-culture experiments using bacteria and mutants of temperate phages that are locked in the lytic cycle have shown that CRISPR–Cas systems can efficiently eliminate the invading phages2,3. Here we show that, when challenged with wild-type temperate phages (which can become lysogenic), type I CRISPR–Cas immune systems cannot eliminate the phages from the bacterial population. Furthermore, our data suggest that, in this context, CRISPR–Cas immune systems are maladaptive to the host, owing to the severe immunopathological effects that are brought about by imperfect matching of spacers to the integrated phage sequences (prophages). These fitness costs drive the loss of CRISPR–Cas from bacterial populations, unless the phage carries anti-CRISPR (acr) genes that suppress the immune system of the host. Using bioinformatics, we show that this imperfect targeting is likely to occur frequently in nature. These findings help to explain the patchy distribution of CRISPR–Cas immune systems within and between bacterial species, and highlight the strong selective benefits of phage-encoded acr genes for both the phage and the host under these circumstances.
中文翻译:

温带噬菌体靶向导致 I 型 CRISPR-Cas 系统丧失
在感染宿主后,感染细菌(噬菌体;以下称为噬菌体)的温带病毒进入裂解或溶原循环。前者导致细菌细胞裂解和噬菌体释放(导致水平传播),而溶原性的特点是噬菌体整合到宿主基因组中,以及休眠(导致垂直传播)1。之前使用细菌和锁定在裂解循环中的温带噬菌体突变体进行的共培养实验表明,CRISPR-Cas 系统可以有效消除入侵的噬菌体2,3。在这里,我们表明,当受到野生型温带噬菌体(可以成为溶原性)的攻击时,I 型 CRISPR-Cas 免疫系统无法消除细菌群体中的噬菌体。此外,我们的数据表明,在这种情况下,CRISPR-Cas免疫系统对宿主适应不良,这是由于间隔区与整合噬菌体序列(原噬菌体)的不完美匹配所带来的严重的免疫病理学效应。这些适应性成本会导致细菌群体中 CRISPR-Cas 的丢失,除非噬菌体携带抑制宿主免疫系统的抗 CRISPR (acr) 基因。利用生物信息学,我们表明这种不完美的靶向很可能在自然界中经常发生。这些发现有助于解释 CRISPR-Cas 免疫系统在细菌物种内部和物种之间的不均匀分布,并强调了噬菌体编码的 acr 基因在这种情况下对噬菌体和宿主的强大选择性优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号