International Journal of Medical Microbiology ( IF 4.5 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.ijmm.2020.151396 Neal Toewiwat 1 , Wirongrong Whangsuk 2 , Poonsakdi Ploypradith 3 , Skorn Mongkolsuk 4 , Suvit Loprasert 4
|
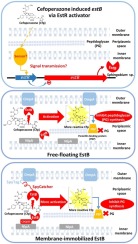
The occurrence of antibiotic resistance bacteria has become a major threat to public health. We have recently discovered a transcriptional activator that belongs to MarR family, EstR, and an esterase B (EstB) with a newly proposed de-arenethiolase activity from Sphingobium sp. SM42. De-arenethiolase activity involves the removal of the small aromatic side chain of cephalosporin antibiotics as an excellent leaving group by the enzymatic C S bond cleavage. Here, we report the regulation of estB through EstR as an activator in response to a third generation cephalosporin, cefoperazone, antibiotic. Cefoperazone induced the expression of estB in wild type Sphingobium sp., but not in the estR knockout strain, and the induction was restored in the complemented strain. Moreover, we revealed the importance of EstB localization in periplasm. Since EsB has the ability to inactivate selected β-lactam antibiotics in vitro, it is possible that the enzyme works at the periplasmic space of Gram negative bacteria similar to β-lactamases. EstB was genetically engineered by incorporating NlpA binding motif, or OmpA signal sequence, or SpyTag-SpyCatcher to the estB gene to mobilize it to different compartments of periplasm; inner membrane, outer membrane, and periplasmic space, respectively. Surprisingly, we found that Sphingobium sp. SM42 and E. coli expressing EstB at the periplasm were more sensitive to cefoperazone. The possible drug enhancement mechanism by enzyme was proposed. This work might lead to a novel strategy to tackle antibiotic resistance problem.
S bond cleavage. Here, we report the regulation of estB through EstR as an activator in response to a third generation cephalosporin, cefoperazone, antibiotic. Cefoperazone induced the expression of estB in wild type Sphingobium sp., but not in the estR knockout strain, and the induction was restored in the complemented strain. Moreover, we revealed the importance of EstB localization in periplasm. Since EsB has the ability to inactivate selected β-lactam antibiotics in vitro, it is possible that the enzyme works at the periplasmic space of Gram negative bacteria similar to β-lactamases. EstB was genetically engineered by incorporating NlpA binding motif, or OmpA signal sequence, or SpyTag-SpyCatcher to the estB gene to mobilize it to different compartments of periplasm; inner membrane, outer membrane, and periplasmic space, respectively. Surprisingly, we found that Sphingobium sp. SM42 and E. coli expressing EstB at the periplasm were more sensitive to cefoperazone. The possible drug enhancement mechanism by enzyme was proposed. This work might lead to a novel strategy to tackle antibiotic resistance problem.
中文翻译:

头孢哌酮通过EstR诱导酯酶B表达,酯酶B增强周质中的头孢哌酮活性。
抗生素抗性细菌的出现已成为对公共卫生的主要威胁。我们最近发现了一种转录激活因子,该转录激活因子属于MarR家族,EstR和酯酶B(EstB),具有新提出的鞘氨醇单孢菌(Sphingobium sp。)的脱-花生四烯酸酶活性。SM42。脱-花生四烯酸酶活性涉及通过酶促CS 键裂解除去头孢菌素抗生素的小的芳香侧链作为极好的离去基团。在这里,我们报道了通过EstR作为对第三代头孢菌素,头孢哌酮,抗生素的激活剂而对estB的调节。头孢哌酮诱导野生型鞘氨醇单胞菌属中的estB表达,但在estR中不表达敲除菌株,并在互补菌株中恢复了诱导。此外,我们揭示了EstB定位在周质中的重要性。由于EsB具有体外灭活所选β-内酰胺抗生素的能力,因此该酶可能在革兰氏阴性细菌的周质空间中起作用,类似于β-内酰胺酶。通过将NlpA结合基序或OmpA信号序列或SpyTag-SpyCatcher掺入estB基因,以将其动员至周质的不同区室,从而对EstB进行了基因工程改造。内膜,外膜和周质空间。令人惊讶的是,我们发现了鞘氨醇单胞菌。SM42和大肠杆菌在周质中表达EstB的人对头孢哌酮更敏感。提出了可能的酶促药物作用机理。这项工作可能会导致解决抗生素耐药性问题的新策略。
键裂解除去头孢菌素抗生素的小的芳香侧链作为极好的离去基团。在这里,我们报道了通过EstR作为对第三代头孢菌素,头孢哌酮,抗生素的激活剂而对estB的调节。头孢哌酮诱导野生型鞘氨醇单胞菌属中的estB表达,但在estR中不表达敲除菌株,并在互补菌株中恢复了诱导。此外,我们揭示了EstB定位在周质中的重要性。由于EsB具有体外灭活所选β-内酰胺抗生素的能力,因此该酶可能在革兰氏阴性细菌的周质空间中起作用,类似于β-内酰胺酶。通过将NlpA结合基序或OmpA信号序列或SpyTag-SpyCatcher掺入estB基因,以将其动员至周质的不同区室,从而对EstB进行了基因工程改造。内膜,外膜和周质空间。令人惊讶的是,我们发现了鞘氨醇单胞菌。SM42和大肠杆菌在周质中表达EstB的人对头孢哌酮更敏感。提出了可能的酶促药物作用机理。这项工作可能会导致解决抗生素耐药性问题的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号