当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning oxygen vacancy concentration of MnO2 through metal doping for improved toluene oxidation.
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-01-22 , DOI: 10.1016/j.jhazmat.2020.122181 Cui Dong 1 , Zhenping Qu 1 , Xiao Jiang 1 , Yewei Ren 1
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-01-22 , DOI: 10.1016/j.jhazmat.2020.122181 Cui Dong 1 , Zhenping Qu 1 , Xiao Jiang 1 , Yewei Ren 1
Affiliation
|
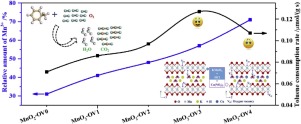
Oxygen vacancy acts an important role in adjusting the chemical properties of MnO2. In this paper, two-dimensional MnO2 catalysts with different oxygen vacancy concentration are obtained by doping Cu2+. It is researched that the K+ species in the interlayer of birnessite-type MnO2 can be substituted during the Cu2+ doping process. Meanwhile, this process will generate the oxygen vacancy. Interestingly, the formation of an appropriate numbers of oxygen vacancy in MnO2 distinctly enhances the low-temperature reducibility and oxygen species activity, which improves the catalytic activity for the toluene oxidation (T100 = 220 °C, Ea=43.6 kJ/mol). However, an excessive concentration of oxygen vacancy in MnO2 sample performs against the activity improvement for toluene oxidation. In situ DRIFTS are applied to elucidate the main intermediates and conversion pathway on MnO2-OV3 with moderate concentration of oxygen vacancy. The results demonstrate that the adsorbed toluene can interact with oxygen species of catalyst to form physisorbed benzaldehyde, aldehydic adsorbate and benzoate species. In addition, it is found that the oxygen vacancy concentration plays an important effect on the oxidation of benzoate species owing to the acceleration effect of oxygen vacancy in the activation of gaseous oxygen.
中文翻译:

通过金属掺杂调节MnO2的氧空位浓度,以改善甲苯的氧化。
氧空位在调节MnO2的化学性质中起重要作用。本文通过掺杂Cu2 +获得了不同氧空位浓度的二维MnO2催化剂。研究表明,在Cu2 +掺杂过程中,水钠锰矿型MnO2中间层中的K +可以被取代。同时,该过程将产生氧空位。有趣的是,在MnO2中形成适当数量的氧空位明显增强了低温还原性和氧物种活性,从而提高了对甲苯氧化的催化活性(T100 = 220°C,Ea = 43.6 kJ / mol)。但是,MnO2样品中氧空位浓度过高不利于甲苯氧化活性的提高。原位DRIFTS用于阐明中等中间体氧空位的MnO2-OV3上的主要中间体和转化途径。结果表明,吸附的甲苯可与催化剂中的氧相互作用,形成物理吸附的苯甲醛,醛吸附物和苯甲酸盐。另外,发现由于气态氧的活化中氧空位的促进作用,氧空位浓度对苯甲酸酯类的氧化起重要作用。
更新日期:2020-01-22
中文翻译:

通过金属掺杂调节MnO2的氧空位浓度,以改善甲苯的氧化。
氧空位在调节MnO2的化学性质中起重要作用。本文通过掺杂Cu2 +获得了不同氧空位浓度的二维MnO2催化剂。研究表明,在Cu2 +掺杂过程中,水钠锰矿型MnO2中间层中的K +可以被取代。同时,该过程将产生氧空位。有趣的是,在MnO2中形成适当数量的氧空位明显增强了低温还原性和氧物种活性,从而提高了对甲苯氧化的催化活性(T100 = 220°C,Ea = 43.6 kJ / mol)。但是,MnO2样品中氧空位浓度过高不利于甲苯氧化活性的提高。原位DRIFTS用于阐明中等中间体氧空位的MnO2-OV3上的主要中间体和转化途径。结果表明,吸附的甲苯可与催化剂中的氧相互作用,形成物理吸附的苯甲醛,醛吸附物和苯甲酸盐。另外,发现由于气态氧的活化中氧空位的促进作用,氧空位浓度对苯甲酸酯类的氧化起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号