当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integrating the (311) facet of MnO2 and the fuctional groups of poly(m-phenylenediamine) in core-shell MnO2@poly(m-phenylenediamine) adsorbent to remove Pb ions from water.
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.jhazmat.2020.122154 Ting Xiong 1 , Xingzhong Yuan 1 , Hou Wang 1 , Longbo Jiang 1 , Zhibin Wu 2 , Han Wang 1 , Xuyang Cao 3
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.jhazmat.2020.122154 Ting Xiong 1 , Xingzhong Yuan 1 , Hou Wang 1 , Longbo Jiang 1 , Zhibin Wu 2 , Han Wang 1 , Xuyang Cao 3
Affiliation
|
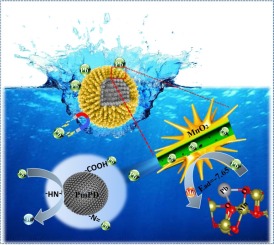
Exposed active facets and functional groups are critical for adsorbents obtaining excellent adsorption properties. In the present study, MnO2@PmPD with exposed active facets was successfully prepared. MnO2,which came from KMnO4 by the sacrificial reductant of PmPD, deposited on the surface of PmPD. Meanwhile, we combined experimental study and theoretical calculations to elucidate the distinct adsorption nature of MnO2@PmPD towards Pb. The surface adsorption of MnO2@PmPD toward Pb was achieved by the interaction between Pb and O atoms on the surface of MnO2. The DFT calculations revealed the facet-dependent adsorption of MnO2 toward Pb. The adsorption affinity of facets toward Pb was in the order of (311) > (111) > (400) > (440), and (311) facet was predominantly adsorption site for Pb. The analysis of partial density of state revealed the strong hybridization between the Pb-p state and O-p states of MnO2. Additionally, the pores of MnO2 provide the interstitial channels for the transportation of Pb into PmPD. The Pb entered the internal of MnO2@PmPD was bonded by the amine and newly formed carboxy groups on PmPD. This study not only develops an efficient adsorbent for heavy metals removing, but also throws light on exemplifying the interaction of Pb with MnO2 based materials.
中文翻译:

将MnO2的(311)面和聚(间苯二胺)的官能团整合到核-壳MnO2 @聚(间苯二胺)吸附剂中,以从水中去除Pb离子。
暴露的活性面和官能团对于获得优异的吸附性能的吸附剂至关重要。在本研究中,成功制备了具有暴露的活性面的MnO2 @ PmPD。MnO2沉积在PmPD的表面,MnO2是通过PmPD的牺牲性还原剂来自KMnO4的。同时,我们结合实验研究和理论计算,阐明了MnO2 @ PmPD对Pb的独特吸附性质。MnO2 @ PmPD对Pb的表面吸附是通过MnO2表面的Pb与O原子之间的相互作用实现的。DFT计算显示MnO2对Pb的刻面依赖性吸附。小平面对Pb的吸附亲和力为(311)>(111)>(400)>(440),并且(311)小平面主要是Pb的吸附位点。对部分状态密度的分析表明,MnO2的Pb-p状态与Op状态之间存在强杂交。此外,MnO2的孔为Pb进入PmPD的运输提供了间隙通道。进入MnO2 @ PmPD内部的Pb被胺和PmPD上新形成的羧基键合。这项研究不仅开发了一种有效的重金属去除吸附剂,而且还为例证Pb与MnO2基材料之间的相互作用提供了例证。
更新日期:2020-01-22
中文翻译:

将MnO2的(311)面和聚(间苯二胺)的官能团整合到核-壳MnO2 @聚(间苯二胺)吸附剂中,以从水中去除Pb离子。
暴露的活性面和官能团对于获得优异的吸附性能的吸附剂至关重要。在本研究中,成功制备了具有暴露的活性面的MnO2 @ PmPD。MnO2沉积在PmPD的表面,MnO2是通过PmPD的牺牲性还原剂来自KMnO4的。同时,我们结合实验研究和理论计算,阐明了MnO2 @ PmPD对Pb的独特吸附性质。MnO2 @ PmPD对Pb的表面吸附是通过MnO2表面的Pb与O原子之间的相互作用实现的。DFT计算显示MnO2对Pb的刻面依赖性吸附。小平面对Pb的吸附亲和力为(311)>(111)>(400)>(440),并且(311)小平面主要是Pb的吸附位点。对部分状态密度的分析表明,MnO2的Pb-p状态与Op状态之间存在强杂交。此外,MnO2的孔为Pb进入PmPD的运输提供了间隙通道。进入MnO2 @ PmPD内部的Pb被胺和PmPD上新形成的羧基键合。这项研究不仅开发了一种有效的重金属去除吸附剂,而且还为例证Pb与MnO2基材料之间的相互作用提供了例证。











































 京公网安备 11010802027423号
京公网安备 11010802027423号