Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.cej.2020.124152 Qi-Su Huang , Chen Wang , Wei Wei , Bing-Jie Ni
|
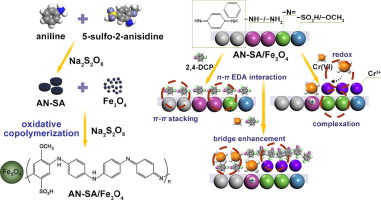
The common coexistence of heavy metal ions (HMIs) and toxic organic matters (OMs) arouses public concerns for their combined toxicity and carcinogenicity. The magnetic poly[aniline(AN)-co-5-sulfo-2-anisidine(SA)] (AN-SA/Fe3O4) was synthesized by an oxidative copolymerization method for the highly−effectively simultaneous removal of Cr(VI) and 2,4-dichlorophenol (2,4-DCP) from aqueous solution. The novel adsorbent exhibited ultra−strong adsorption capacities for sole Cr(VI) and sole 2,4-DCP. The mechanism studies revealed that Cr(VI) species (HCrO4- and Cr2O72- in solution pH as 5) were reduced to Cr(III) by the −NH−/−NH2 groups after attaching to the protonated binding sites of AN-SA/Fe3O4 through electrostatic attraction. By contrast, multiple reactions involving the n-π electron donor−acceptor (EDA) interaction, π-π stacking and hydrogen bond contributed to the elimination of 2,4-DCP. In binary system, the coexistent Cr(VI) and 2,4-DCP elevated mutual adsorption capacities by 88.1% and 102.1%, respectively. Specially, 2,4-DCP can form bridge interactions with both Cr(VI) and Cr(III) due to conjugate effect. This property enabled Cr(VI) to additionally link to the hydrophobic sites, except for the hydrophilic sites, via 2,4-DCP bridges. Moreover, the produced Cr(III) can forcefully captured 2,4-DCP with the electron−rich groups (i.e., −NH−, −N =, −SO3H, −OCH3) on AN-SA/Fe3O4 to form the multi−components complexes. The bridge interactions (i.e., n-π EDA interaction, complexation) created the newly available sites for Cr(VI) and 2,4-DCP, resulting in enlarged adsorbance and synchronous removal on AN-SA/Fe3O4 in coexisting system. In addition, the high proportion of –N= groups generated by Cr(VI) oxidation also devoted to the uptake enhancement due to its strong affinity for 2,4-DCP. Overall, the high−performance and synergistic removal qualified AN-SA/Fe3O4 as a multifunctional adsorbent for the integrated treatment of HIMs-OMs hybrid pollutants.
中文翻译:

磁性聚(苯胺-共-5-磺基-2-甲氧基苯胺)作为多功能吸附剂用于高效共同去除水的Cr(VI)和2,4-二氯酚
重金属离子(HMI)和有毒有机物(OM)的共存引起了公众对其毒性和致癌性的关注。磁性聚[苯胺(AN) -共-5-磺基-2-甲氧基苯胺(SA)](AN-SA / Fe的3 ö 4)通过对所述高度有效地同时去除的Cr的氧化共聚方法(合成VI )和2,4-二氯苯酚(2,4-DCP)的水溶液。新型吸附剂对唯一的Cr(VI)和唯一的2,4-DCP表现出超强的吸附能力。机制研究表明,铬(VI)物种(HCRO 4 -和Cr 2 ö 7 2-在溶液的pH为5)由-NH还原为Cr(III) - / - NH 2通过静电吸引连接到AN-SA / Fe 3 O 4的质子化结合位点后的基团。相比之下,涉及n-π电子供体-受体(EDA)相互作用的多个反应π-π堆积和氢键有助于消除2,4-DCP。在二元体系中,共存的Cr(VI)和2,4-DCP相互吸附能力分别提高了88.1%和102.1%。特别地,由于共轭效应,2,4-DCP可以与Cr(VI)和Cr(III)形成桥相互作用。此特性使Cr(VI)可以通过2,4-DCP桥连接除亲水位点以外的疏水位点。此外,生成的Cr(III)可以在AN-SA / Fe 3 O上强力捕获带有富电子基团(即-NH-,-N =,-SO 3 H,-OCH 3)的2,4-DCP 4形成多组分复合物。桥相互作用(即,n-πEDA相互作用,络合作用)为Cr(VI)和2,4-DCP创建了新的可用位点,导致在共存系统中对AN-SA / Fe 3 O 4的吸附增大并同步去除。此外,由于其对2,4-DCP的强亲和力,由Cr(VI)氧化生成的高比例的–N =基团也专门用于吸收增强。总体而言,高性能和协同去除合格的AN-SA / Fe 3 O 4是用于HIMs-OMs混合污染物综合处理的多功能吸附剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号