Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2020-01-22 , DOI: 10.1016/j.micromeso.2020.110039 Arnab Ghosh , Gopal Das
|
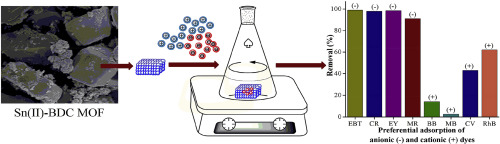
Tin metal-organic framework [Sn(II)-BDC MOF] was synthesized following the hydrothermal synthesis route in environmentally benign conditions. The material was applied for preferential and efficient removal of toxic anionic dyes viz. CR, EBT, and EY from aqueous solution. The thermal and aqueous stability of the material was confirmed using PXRD and FTIR analysis. Adsorption kinetic study and the thermodynamic parameters were also investigated. A better fit with the second-order kinetic model indicated that the process is predominantly chemisorption. The Langmuir and Freundlich isotherm models were used for validating the experimental data, where the Langmuir model provided a good fit. The maximum adsorption capacity (qm) for three anionic dyes (CR, EBT, and EY) was 95.2 mg g1, 125 mg g-1, and 208.3 mg g-1, respectively. The actual evaluation of the adsorption performance was based on the partition coefficient (PC) values. At an initial concentration of 100 mg L-1, the PC values for CR, EBT, and EY were 33.79, 30.29, and 40.44 mg g-1 μM-1, respectively. Based on high PC values and maximum adsorption capacity, Sn(II)-BDC MOF turned out to be an excellent material for the removal of anionic dyes. Further, retention of > 86% of its adsorption efficiency after three adsorption-desorption cycles indicated the potential reusability of the synthesized material. This study provides valuable insight into the eco-friendly synthesis of a novel water-stable Sn(II)-BDC MOF and its application as a promising adsorbent in the removal of toxic anionic dyes from aqueous solutions.
中文翻译:

Sn(II)-BDC MOF的绿色合成:阴离子染料的优选吸附
锡金属有机骨架[Sn(II)-BDC MOF]是在环境良好的条件下按照水热合成路线合成的。该材料用于优先有效地去除有毒阴离子染料。水溶液中的CR,EBT和EY。使用PXRD和FTIR分析确认了材料的热稳定性和水稳定性。还研究了吸附动力学和热力学参数。与二阶动力学模型的更好拟合表明该过程主要是化学吸附。Langmuir和Freundlich等温线模型用于验证实验数据,其中Langmuir模型提供了很好的拟合度。三种阴离子染料(CR,EBT和EY)的最大吸附容量(q m)为95.2 mg g1,125毫克克-1,和208.3毫克克-1,分别。吸附性能的实际评估基于分配系数(PC)值。在100毫克的L的初始浓度-1,对于CR,EBT,和EY的PC值分别为33.79,30.29,40.44和毫克克-1 μM -1, 分别。基于高的PC值和最大的吸附能力,Sn(II)-BDC MOF被证明是一种用于去除阴离子染料的极佳材料。此外,在三个吸附-解吸循环后,其吸附效率保持> 86%,表明合成材料具有潜在的可重复使用性。这项研究为新型水稳定性Sn(II)-BDC MOF的生态友好合成及其作为从水溶液中去除有毒阴离子染料的有前途的吸附剂的应用提供了宝贵的见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号