当前位置:
X-MOL 学术
›
Mol. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sirt1-Overexpressing Mesenchymal Stem Cells Drive the Anti-tumor Effect through Their Pro-inflammatory Capacity.
Molecular Therapy ( IF 12.1 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.ymthe.2020.01.018 Fei Ye 1 , Jinghua Jiang 1 , Chen Zong 1 , Xue Yang 1 , Lu Gao 1 , Yan Meng 1 , Rong Li 1 , Qiudong Zhao 1 , Zhipeng Han 1 , Lixin Wei 1
Molecular Therapy ( IF 12.1 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.ymthe.2020.01.018 Fei Ye 1 , Jinghua Jiang 1 , Chen Zong 1 , Xue Yang 1 , Lu Gao 1 , Yan Meng 1 , Rong Li 1 , Qiudong Zhao 1 , Zhipeng Han 1 , Lixin Wei 1
Affiliation
|
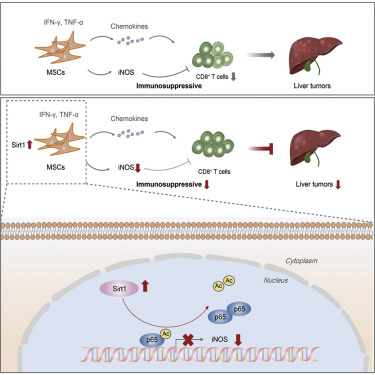
The major obstacles for the efficacy of tumor immunotherapies are their immune-related systemic adverse events. Therefore, tumor tropism property and pro-inflammatory ability of mesenchymal stem cells (MSCs) could be utilized in combination to potentiate local immunity for cancer eradication. We previously observed that MSCs with the type III histone deacetylase silent information regulator 2 homologue 1 (Sirt1) overexpression displayed a pro-inflammatory capacity. However, the anti-tumor effect of Sirt1-overexpressing MSCs and the role of Sirt1 in regulating the pro-inflammatory capacity of MSCs still need to be clarified. In this study, utilizing the hepatic metastasis model of colorectal carcinoma, we demonstrated that Sirt1-overexpressing MSCs significantly exerted anti-tumor activity through increasing the number of CD8+ T cells. Furthermore, Sirt1 did not affect chemokine secretion in MSCs induced by inflammatory cytokines, but impaired the immunosuppressive ability of MSCs through suppressing inflammatory cytokine-stimulated inducible nitric oxide synthase (iNOS) production via deacetylating p65. iNOS overexpression negated the anti-tumor effect of Sirt1-overexpressing MSCs. Collectively, our data defined Sirt1 as the critical regulator for modulating the pro-inflammatory ability of MSCs, and they suggested that Sirt1-overexpressing MSCs secreting chemokines but little iNOS under the inflammatory milieu were capable of attracting immune cells to close proximity without suppressing their proliferation, thereby achieving a potent anti-tumor effect.
中文翻译:

Sirt1过表达的间充质干细胞通过其促炎能力驱动抗肿瘤作用。
肿瘤免疫疗法功效的主要障碍是其免疫相关的全身性不良事件。因此,可以将间质干细胞(MSCs)的肿瘤嗜性和促炎能力结合起来,以增强局部免疫力,从而根除癌症。我们以前观察到,具有III型组蛋白脱乙酰基酶沉默信息调节剂2同源物1(Sirt1)过度表达的MSC表现出促炎能力。然而,仍然需要弄清楚Sirt1过表达的MSCs的抗肿瘤作用和Sirt1在调节MSCs的促炎能力中的作用。在这项研究中,利用大肠癌的肝转移模型,我们证明了Sirt1过表达的MSC通过增加CD8 + T细胞的数量显着发挥了抗肿瘤活性。此外,Sirt1不会影响炎症细胞因子诱导的MSC中趋化因子的分泌,但是通过抑制炎症细胞因子刺激的一氧化氮合酶(iNOS)产生(通过使p65脱乙酰化)来抑制MSC的免疫抑制能力。iNOS的过量表达消除了Sirt1过量表达的MSC的抗肿瘤作用。总的来说,我们的数据将Sirt1定义为调节MSCs促炎能力的关键调节剂,他们认为,表达Sirt1的MSCs分泌趋化因子,但在炎症环境下几乎没有iNOS能够吸引免疫细胞紧密结合而不抑制其增殖。 ,从而达到有效的抗肿瘤作用。但是通过抑制炎性细胞因子刺激的可诱导型一氧化氮合酶(iNOS)通过使p65脱乙酰化而产生,从而削弱了MSC的免疫抑制能力。iNOS的过量表达消除了Sirt1过量表达的MSC的抗肿瘤作用。总的来说,我们的数据将Sirt1定义为调节MSCs促炎能力的关键调节剂,他们认为,表达Sirt1的MSCs分泌趋化因子,但在炎症环境下几乎没有iNOS能够吸引免疫细胞紧密结合而不抑制其增殖。 ,从而达到有效的抗肿瘤作用。但是通过抑制炎性细胞因子刺激的可诱导型一氧化氮合酶(iNOS)通过使p65脱乙酰化而产生,从而削弱了MSC的免疫抑制能力。iNOS的过量表达消除了Sirt1过量表达的MSC的抗肿瘤作用。总的来说,我们的数据将Sirt1定义为调节MSCs促炎能力的关键调节剂,他们认为,表达Sirt1的MSCs分泌趋化因子,但在炎症环境下几乎没有iNOS能够吸引免疫细胞紧密结合而不抑制其增殖。 ,从而达到有效的抗肿瘤作用。
更新日期:2020-01-22
中文翻译:

Sirt1过表达的间充质干细胞通过其促炎能力驱动抗肿瘤作用。
肿瘤免疫疗法功效的主要障碍是其免疫相关的全身性不良事件。因此,可以将间质干细胞(MSCs)的肿瘤嗜性和促炎能力结合起来,以增强局部免疫力,从而根除癌症。我们以前观察到,具有III型组蛋白脱乙酰基酶沉默信息调节剂2同源物1(Sirt1)过度表达的MSC表现出促炎能力。然而,仍然需要弄清楚Sirt1过表达的MSCs的抗肿瘤作用和Sirt1在调节MSCs的促炎能力中的作用。在这项研究中,利用大肠癌的肝转移模型,我们证明了Sirt1过表达的MSC通过增加CD8 + T细胞的数量显着发挥了抗肿瘤活性。此外,Sirt1不会影响炎症细胞因子诱导的MSC中趋化因子的分泌,但是通过抑制炎症细胞因子刺激的一氧化氮合酶(iNOS)产生(通过使p65脱乙酰化)来抑制MSC的免疫抑制能力。iNOS的过量表达消除了Sirt1过量表达的MSC的抗肿瘤作用。总的来说,我们的数据将Sirt1定义为调节MSCs促炎能力的关键调节剂,他们认为,表达Sirt1的MSCs分泌趋化因子,但在炎症环境下几乎没有iNOS能够吸引免疫细胞紧密结合而不抑制其增殖。 ,从而达到有效的抗肿瘤作用。但是通过抑制炎性细胞因子刺激的可诱导型一氧化氮合酶(iNOS)通过使p65脱乙酰化而产生,从而削弱了MSC的免疫抑制能力。iNOS的过量表达消除了Sirt1过量表达的MSC的抗肿瘤作用。总的来说,我们的数据将Sirt1定义为调节MSCs促炎能力的关键调节剂,他们认为,表达Sirt1的MSCs分泌趋化因子,但在炎症环境下几乎没有iNOS能够吸引免疫细胞紧密结合而不抑制其增殖。 ,从而达到有效的抗肿瘤作用。但是通过抑制炎性细胞因子刺激的可诱导型一氧化氮合酶(iNOS)通过使p65脱乙酰化而产生,从而削弱了MSC的免疫抑制能力。iNOS的过量表达消除了Sirt1过量表达的MSC的抗肿瘤作用。总的来说,我们的数据将Sirt1定义为调节MSCs促炎能力的关键调节剂,他们认为,表达Sirt1的MSCs分泌趋化因子,但在炎症环境下几乎没有iNOS能够吸引免疫细胞紧密结合而不抑制其增殖。 ,从而达到有效的抗肿瘤作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号