当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient solid amino acid–clinoptilolite nanoparticles adsorbent for Mn(II) removal: A comprehensive study on designing the experiments, thermodynamic and kinetic aspects
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.solidstatesciences.2020.106124 Meymanat Mehrali-Afjani , Alireza Nezamzadeh-Ejhieh
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.solidstatesciences.2020.106124 Meymanat Mehrali-Afjani , Alireza Nezamzadeh-Ejhieh
|
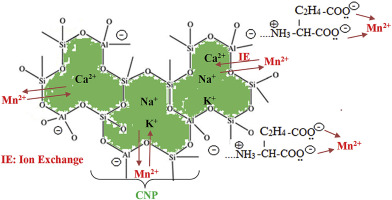
Abstract Glutamic acid-clinoptilolite nanoparticles (GA-CNP) was used for the Mn(II) removal in aquatic media. XRD, FT-IR, SEM, TG analyzer and energy dispersive analysis X-ray spectroscopy (EDX) were used for the characterization of the raw and the modified samples. Initial experiments showed a maximum adsorption capacity of 0.572 mmol Mn/g for the raw CNP while the modified CNP-GA showed a value of 0.691 mmol Mn/g, confirming the positive role of the CNP modification in Mn(II) removal. Designing the experiments by response surface methodology (RSM) showed a quadratic model for the processing of the data obtained in the removal process. The optimum conditions to achieve maximum adsorption were suggested as initial Mn(II) concentration: 583 mg/L, contact time: 132 min, CNP-GA dosage: 5 g L−1 and manganese solution pH: 3.5. The importance of each term was calculated by the Parreto analysis and the results showed that the singular factors have the sequence of adsorbent dosage (37.4%)>CMn (24.1%) >contacting time (2.2%) which agrees with the sequence obtained by the F-values. For the terms containing interaction effects the most importance percentages of 11.1% and 7% obtained for time-dosage and pH-CMn, respectively. Among the quadratic terms, the most percentage of 9.8% was obtained for (CMn)2. Adsorption isotherms of Mn(II) well modeled by Langmuir equation, showing a monolayer sorption of Mn(II), while a pseudo-second-order rate equation well modeled the kinetics of the process, confirming a chemical reaction limits the rate of the removal process.
中文翻译:

用于去除锰(II)的高效固体氨基酸-斜发沸石纳米颗粒吸附剂:对实验设计、热力学和动力学方面的综合研究
摘要 谷氨酸-斜发沸石纳米颗粒 (GA-CNP) 用于去除水介质中的 Mn(II)。XRD、FT-IR、SEM、TG 分析仪和能量色散分析 X 射线光谱 (EDX) 用于表征原始样品和改性样品。初始实验表明,原始 CNP 的最大吸附容量为 0.572 mmol Mn/g,而改性 CNP-GA 显示值为 0.691 mmol Mn/g,证实了 CNP 改性在去除 Mn(II) 中的积极作用。通过响应面方法 (RSM) 设计实验显示了用于处理去除过程中获得的数据的二次模型。建议实现最大吸附的最佳条件为初始锰(II)浓度:583 mg/L,接触时间:132 分钟,CNP-GA 用量:5 g L-1 和锰溶液 pH:3.5。Parreto分析计算了每一项的重要性,结果表明奇异因子的顺序为吸附剂用量(37.4%)>CMn(24.1%)>接触时间(2.2%),与通过分析得到的顺序一致。 F 值。对于包含相互作用影响的术语,时间剂量和 pH-CMn 的最重要百分比分别为 11.1% 和 7%。在二次项中,(CMn)2 的百分比最高,为 9.8%。Mn(II) 的吸附等温线由 Langmuir 方程很好地模拟,显示了 Mn(II) 的单层吸附,而伪二级速率方程很好地模拟了该过程的动力学,证实了化学反应限制了去除速率过程。
更新日期:2020-03-01
中文翻译:

用于去除锰(II)的高效固体氨基酸-斜发沸石纳米颗粒吸附剂:对实验设计、热力学和动力学方面的综合研究
摘要 谷氨酸-斜发沸石纳米颗粒 (GA-CNP) 用于去除水介质中的 Mn(II)。XRD、FT-IR、SEM、TG 分析仪和能量色散分析 X 射线光谱 (EDX) 用于表征原始样品和改性样品。初始实验表明,原始 CNP 的最大吸附容量为 0.572 mmol Mn/g,而改性 CNP-GA 显示值为 0.691 mmol Mn/g,证实了 CNP 改性在去除 Mn(II) 中的积极作用。通过响应面方法 (RSM) 设计实验显示了用于处理去除过程中获得的数据的二次模型。建议实现最大吸附的最佳条件为初始锰(II)浓度:583 mg/L,接触时间:132 分钟,CNP-GA 用量:5 g L-1 和锰溶液 pH:3.5。Parreto分析计算了每一项的重要性,结果表明奇异因子的顺序为吸附剂用量(37.4%)>CMn(24.1%)>接触时间(2.2%),与通过分析得到的顺序一致。 F 值。对于包含相互作用影响的术语,时间剂量和 pH-CMn 的最重要百分比分别为 11.1% 和 7%。在二次项中,(CMn)2 的百分比最高,为 9.8%。Mn(II) 的吸附等温线由 Langmuir 方程很好地模拟,显示了 Mn(II) 的单层吸附,而伪二级速率方程很好地模拟了该过程的动力学,证实了化学反应限制了去除速率过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号