当前位置:
X-MOL 学术
›
Eur. J. Pharm. Biopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Validation of hyaluronic acid-agar-based hydrogels as vitreous humor mimetics for in vitro drug and particle migration evaluations.
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2020-01-22 , DOI: 10.1016/j.ejpb.2020.01.008 Sachin S Thakur 1 , Siddharth K Shenoy 2 , Jung Soo Suk 3 , Justin S Hanes 4 , Ilva D Rupenthal 5
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2020-01-22 , DOI: 10.1016/j.ejpb.2020.01.008 Sachin S Thakur 1 , Siddharth K Shenoy 2 , Jung Soo Suk 3 , Justin S Hanes 4 , Ilva D Rupenthal 5
Affiliation
|
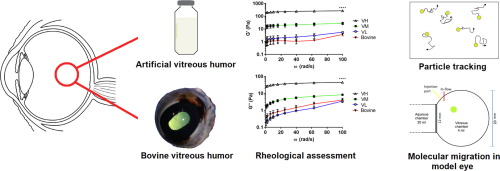
Artificial vitreous humor holds immense potential for use in in vitro intravitreal drug delivery assays. In this study, we investigated rheological properties and drug or nanoparticle migration in hyaluronic acid (HA) - agar based hydrogels and compared these characteristics with bovine vitreous humor. Gel compositions identified in literature containing HA (0.7-5.0 mg/ml) and agar (0.95-4.0 mg/ml) were classified as either high (VH), medium (VM) or low (VL) polymer load. Viscoelastic behavior was evaluated using oscillatory rheology, and migration of differently sized and charged polystyrene nanoparticles (NPs) through the different gels was determined via multiple particle tracking. Comparable rheological behaviour was observed between VL and bovine vitreous. Tracking evaluations revealed that increasing particle size and gel viscosity slowed NP migration. Additionally, 100 nm anionic NPs migrated slower than neutral NPs in VL and VM, while cationic NPs were immobile in all gels. Finally, distribution and clearance of sodium fluorescein was used to model drug mobility through the gels using a custom-built eye model. Flow and angular movement only influenced drug migration in VL and VM, but not VH. Finally, VL and VM demonstrated to have the most similar sodium fluorescein clearance to that of bovine vitreous humor. Together, these evaluations demonstrate that low viscosity HA-agar gels can be used to approximate nanoparticle and drug migration through biological vitreous humor.
中文翻译:

验证透明质酸琼脂基水凝胶作为玻璃体液模拟物的体外药物和颗粒迁移评估。
人工玻璃体液具有用于体外玻璃体内药物递送测定的巨大潜力。在这项研究中,我们研究了透明质酸(HA)-琼脂基水凝胶的流变特性和药物或纳米颗粒迁移,并将这些特征与牛玻璃体液进行了比较。文献中鉴定的含有HA(0.7-5.0 mg / ml)和琼脂(0.95-4.0 mg / ml)的凝胶组合物被分为高(VH),中(VM)或低(VL)聚合物负载量。使用振荡流变学评估粘弹性行为,并通过多次颗粒追踪确定大小不同且带电的聚苯乙烯纳米颗粒(NP)通过不同凝胶的迁移。在VL和牛玻璃体之间观察到可比的流变行为。跟踪评估显示,增加粒径和凝胶粘度会减慢NP迁移。此外,在VL和VM中,100 nm阴离子NPs的迁移速度比中性NPs慢,而阳离子NPs在所有凝胶中均不可移动。最后,使用定制的眼睛模型,使用荧光素钠的分布和清除率来模拟药物在凝胶中的移动性。流量和角运动仅影响VL和VM中的药物迁移,但不影响VH。最终,VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。而阳离子NPs在所有凝胶中均不可移动。最后,使用定制的眼睛模型,使用荧光素钠的分布和清除率来模拟药物在凝胶中的移动性。流量和角运动仅影响VL和VM中的药物迁移,但不影响VH。最终,VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。而阳离子NPs在所有凝胶中均不可移动。最后,使用定制的眼睛模型,使用荧光素钠的分布和清除率来模拟药物在凝胶中的移动性。流量和角运动仅影响VL和VM中的药物迁移,但不影响VH。最终,VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。
更新日期:2020-01-22
中文翻译:

验证透明质酸琼脂基水凝胶作为玻璃体液模拟物的体外药物和颗粒迁移评估。
人工玻璃体液具有用于体外玻璃体内药物递送测定的巨大潜力。在这项研究中,我们研究了透明质酸(HA)-琼脂基水凝胶的流变特性和药物或纳米颗粒迁移,并将这些特征与牛玻璃体液进行了比较。文献中鉴定的含有HA(0.7-5.0 mg / ml)和琼脂(0.95-4.0 mg / ml)的凝胶组合物被分为高(VH),中(VM)或低(VL)聚合物负载量。使用振荡流变学评估粘弹性行为,并通过多次颗粒追踪确定大小不同且带电的聚苯乙烯纳米颗粒(NP)通过不同凝胶的迁移。在VL和牛玻璃体之间观察到可比的流变行为。跟踪评估显示,增加粒径和凝胶粘度会减慢NP迁移。此外,在VL和VM中,100 nm阴离子NPs的迁移速度比中性NPs慢,而阳离子NPs在所有凝胶中均不可移动。最后,使用定制的眼睛模型,使用荧光素钠的分布和清除率来模拟药物在凝胶中的移动性。流量和角运动仅影响VL和VM中的药物迁移,但不影响VH。最终,VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。而阳离子NPs在所有凝胶中均不可移动。最后,使用定制的眼睛模型,使用荧光素钠的分布和清除率来模拟药物在凝胶中的移动性。流量和角运动仅影响VL和VM中的药物迁移,但不影响VH。最终,VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。而阳离子NPs在所有凝胶中均不可移动。最后,使用定制的眼睛模型,使用荧光素钠的分布和清除率来模拟药物在凝胶中的移动性。流量和角运动仅影响VL和VM中的药物迁移,但不影响VH。最终,VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。VL和VM被证明具有与牛玻璃体液最相似的荧光素钠清除率。总之,这些评估表明,低粘度HA-琼脂凝胶可用于通过生物玻璃体液近似纳米颗粒和药物的迁移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号