当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water-soluble ZnO quantum dots modified by (3-aminopropyl)triethoxysilane: The promising fluorescent probe for the selective detection of Cu2+ ion in drinking water
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.jallcom.2020.153904 Tong Zou , Xinxin Xing , Yue Yang , Zhezhe Wang , Zidong Wang , Rongjun Zhao , Xu Zhang , Yude Wang
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.jallcom.2020.153904 Tong Zou , Xinxin Xing , Yue Yang , Zhezhe Wang , Zidong Wang , Rongjun Zhao , Xu Zhang , Yude Wang
|
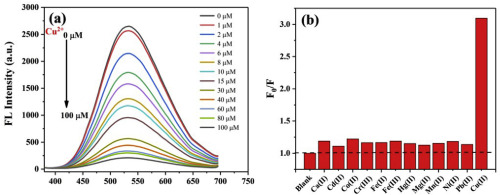
Abstract Copper, as an essential element in human body, can have adverse impact on environment and healthy individuals if it is excessive. So it is necessary to establish a rapid and effective method for detecting Cu2+. In this work, we describe a method for determination of Cu2+ based on water-soluble ZnO quantum dots (QDs) modified with (3-aminopropyl)triethoxysilane (APTEs). The ZnO QDs functionalized with APTEs (NH2–ZnO QDs) synthesized by a simple sol-gel method and displayed strong yellow-green fluorescence with a peak at 535 nm under 350 nm excitation. High-resolution transmission electron microscopy, Fourier transform infrared spectroscopy, luminescence, and UV–visible absorption spectroscopy were used to characterize the NH2–ZnO QDs. In addition, the emission from NH2–ZnO QDs was selectively quenched upon addition of Cu2+. Therefore, this finding was used to design a fluorescent probe based on NH2–ZnO QDs to detect Cu2+ in water solution, and the linear relationships were 2–20 nM and 1–100 μM respectively, with detection limit for Cu2+ at 1.72 nM (on the basis of 3σ/slope criterion). This fluorescent probe had also been applied in real water sample to testify its availability in drinking water. Furthermore, the quenching mechanism was studied by measurements of UV–visible absorption spectra and fluorescent lifetime of ZnO QDs, which may be attributed to the aggregation induced by Cu2+ and the dynamic quenching existing energy transfer between QDs and Cu2+.
中文翻译:

(3-氨基丙基)三乙氧基硅烷修饰的水溶性ZnO量子点:用于选择性检测饮用水中Cu2+离子的有前途的荧光探针
摘要 铜作为人体必需的元素,过量使用会对环境和健康产生不利影响。因此有必要建立一种快速有效的Cu2+检测方法。在这项工作中,我们描述了一种基于用(3-氨基丙基)三乙氧基硅烷 (APTE) 改性的水溶性 ZnO 量子点 (QD) 测定 Cu2+ 的方法。通过简单的溶胶-凝胶法合成的 APTE(NH2-ZnO QDs)功能化的 ZnO QDs 显示出强烈的黄绿色荧光,在 350 nm 激发下在 535 nm 处有一个峰值。高分辨率透射电子显微镜、傅里叶变换红外光谱、发光和紫外-可见吸收光谱被用来表征NH2-ZnO QD。此外,NH2-ZnO QD 的发射在添加 Cu2+ 后被选择性淬灭。所以,利用这一发现设计了一种基于 NH2-ZnO QDs 的荧光探针来检测水溶液中的 Cu2+,线性关系分别为 2-20 nM 和 1-100 μM,对 Cu2+ 的检测限为 1.72 nM(基于3σ/斜率准则)。这种荧光探针也已应用于实际水样,以证明其在饮用水中的可用性。此外,通过测量 ZnO QDs 的紫外-可见吸收光谱和荧光寿命来研究猝灭机制,这可能归因于 Cu2+ 诱导的聚集和 QDs 和 Cu2+ 之间存在的能量转移的动态猝灭。72 nM(基于 3σ/斜率标准)。这种荧光探针也已应用于实际水样,以证明其在饮用水中的可用性。此外,通过测量 ZnO QDs 的紫外-可见吸收光谱和荧光寿命来研究猝灭机制,这可能归因于 Cu2+ 诱导的聚集和 QDs 和 Cu2+ 之间存在的能量转移的动态猝灭。72 nM(基于 3σ/斜率标准)。这种荧光探针也已应用于实际水样,以证明其在饮用水中的可用性。此外,通过测量 ZnO QDs 的紫外-可见吸收光谱和荧光寿命来研究猝灭机制,这可能归因于 Cu2+ 诱导的聚集和 QDs 和 Cu2+ 之间存在的能量转移的动态猝灭。
更新日期:2020-06-01
中文翻译:

(3-氨基丙基)三乙氧基硅烷修饰的水溶性ZnO量子点:用于选择性检测饮用水中Cu2+离子的有前途的荧光探针
摘要 铜作为人体必需的元素,过量使用会对环境和健康产生不利影响。因此有必要建立一种快速有效的Cu2+检测方法。在这项工作中,我们描述了一种基于用(3-氨基丙基)三乙氧基硅烷 (APTE) 改性的水溶性 ZnO 量子点 (QD) 测定 Cu2+ 的方法。通过简单的溶胶-凝胶法合成的 APTE(NH2-ZnO QDs)功能化的 ZnO QDs 显示出强烈的黄绿色荧光,在 350 nm 激发下在 535 nm 处有一个峰值。高分辨率透射电子显微镜、傅里叶变换红外光谱、发光和紫外-可见吸收光谱被用来表征NH2-ZnO QD。此外,NH2-ZnO QD 的发射在添加 Cu2+ 后被选择性淬灭。所以,利用这一发现设计了一种基于 NH2-ZnO QDs 的荧光探针来检测水溶液中的 Cu2+,线性关系分别为 2-20 nM 和 1-100 μM,对 Cu2+ 的检测限为 1.72 nM(基于3σ/斜率准则)。这种荧光探针也已应用于实际水样,以证明其在饮用水中的可用性。此外,通过测量 ZnO QDs 的紫外-可见吸收光谱和荧光寿命来研究猝灭机制,这可能归因于 Cu2+ 诱导的聚集和 QDs 和 Cu2+ 之间存在的能量转移的动态猝灭。72 nM(基于 3σ/斜率标准)。这种荧光探针也已应用于实际水样,以证明其在饮用水中的可用性。此外,通过测量 ZnO QDs 的紫外-可见吸收光谱和荧光寿命来研究猝灭机制,这可能归因于 Cu2+ 诱导的聚集和 QDs 和 Cu2+ 之间存在的能量转移的动态猝灭。72 nM(基于 3σ/斜率标准)。这种荧光探针也已应用于实际水样,以证明其在饮用水中的可用性。此外,通过测量 ZnO QDs 的紫外-可见吸收光谱和荧光寿命来研究猝灭机制,这可能归因于 Cu2+ 诱导的聚集和 QDs 和 Cu2+ 之间存在的能量转移的动态猝灭。











































 京公网安备 11010802027423号
京公网安备 11010802027423号