Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: A multicountry analysis of cross-sectional data.
PLOS Medicine ( IF 15.8 ) Pub Date : 2020-01-21 , DOI: 10.1371/journal.pmed.1003008 Anna S Dean 1 , Matteo Zignol 1 , Andrea Maurizio Cabibbe 2 , Dennis Falzon 1 , Philippe Glaziou 1 , Daniela Maria Cirillo 2 , Claudio U Köser 3 , Lice Y Gonzalez-Angulo 1 , Olga Tosas-Auget 1 , Nazir Ismail 4 , Sabira Tahseen 5 , Maria Cecilia G Ama 6 , Alena Skrahina 7 , Natavan Alikhanova 8 , S M Mostofa Kamal 9 , Katherine Floyd 1
PLOS Medicine ( IF 15.8 ) Pub Date : 2020-01-21 , DOI: 10.1371/journal.pmed.1003008 Anna S Dean 1 , Matteo Zignol 1 , Andrea Maurizio Cabibbe 2 , Dennis Falzon 1 , Philippe Glaziou 1 , Daniela Maria Cirillo 2 , Claudio U Köser 3 , Lice Y Gonzalez-Angulo 1 , Olga Tosas-Auget 1 , Nazir Ismail 4 , Sabira Tahseen 5 , Maria Cecilia G Ama 6 , Alena Skrahina 7 , Natavan Alikhanova 8 , S M Mostofa Kamal 9 , Katherine Floyd 1
Affiliation
|
BACKGROUND
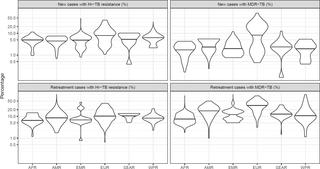
The surveillance of drug resistance among tuberculosis (TB) patients is central to combatting the global TB epidemic and preventing the spread of antimicrobial resistance. Isoniazid and rifampicin are two of the most powerful first-line anti-TB medicines, and resistance to either of them increases the risk of treatment failure, relapse, or acquisition of resistance to other drugs. The global prevalence of rifampicin resistance is well documented, occurring in 3.4% (95% CI 2.5%-4.4%) of new TB patients and 18% (95% CI 7.6%-31%) of previously treated TB patients in 2018, whereas the prevalence of isoniazid resistance at global and regional levels is less understood. In 2018, the World Health Organization (WHO) recommended a modified 6-month treatment regimen for people with isoniazid-resistant, rifampicin-susceptible TB (Hr-TB), which includes rifampicin, pyrazinamide, ethambutol, and levofloxacin. We estimated the global prevalence of Hr-TB among TB patients and investigated associated phenotypic and genotypic drug resistance patterns.
METHODS AND FINDINGS
Aggregated drug resistance data reported to WHO from either routine continuous surveillance or nationally representative periodic surveys of TB patients for the period 2003-2017 were reviewed. Isoniazid data were available from 156 countries or territories for 211,753 patients. Among these, the global prevalence of Hr-TB was 7.4% (95% CI 6.5%-8.4%) among new TB patients and 11.4% (95% CI 9.4%-13.4%) among previously treated TB patients. Additional data on pyrazinamide and levofloxacin resistance were available from 6 countries (Azerbaijan, Bangladesh, Belarus, Pakistan, the Philippines, and South Africa). There were no cases of resistance to both pyrazinamide and levofloxacin among Hr-TB patients, except for the Philippines (1.8%, 95% CI 0.2-6.4) and Belarus (5.3%, 95% CI 0.1-26.0). Sequencing data for all genomic regions involved in isoniazid resistance were available for 4,563 patients. Among the 1,174 isolates that were resistant by either phenotypic testing or sequencing, 78.6% (95% CI 76.1%-80.9%) had resistance-conferring mutations in the katG gene and 14.6% (95% CI 12.7%-16.8%) in both katG and the inhA promoter region. For 6.8% (95% CI 5.4%-8.4%) of patients, mutations occurred in the inhA promoter alone, for whom an increased dose of isoniazid may be considered. The main limitations of this study are that most analyses were performed at the national rather than individual patient level and that the quality of laboratory testing may vary between countries.
CONCLUSIONS
In this study, the prevalence of Hr-TB among TB patients was higher than the prevalence of rifampicin resistance globally. Many patients with Hr-TB would be missed by current diagnostic algorithms driven by rifampicin testing, highlighting the need for new rapid molecular technologies to ensure access to appropriate treatment and care. The low prevalence of resistance to pyrazinamide and fluoroquinolones among patients with Hr-TB provides further justification for the recommended modified treatment regimen.
中文翻译:

结核患者异烟肼耐药性的流行和遗传学特征:横断面数据的多国分析。
背景技术在结核病(TB)患者中对耐药性的监测对于抵抗全球结核病流行和防止抗菌素耐药性的传播至关重要。异烟肼和利福平是最强大的一线抗结核药物中的两种,对它们中任何一种的抗药性都会增加治疗失败,复发或对其他药物产生抗药性的风险。利福平耐药性的全球患病率已得到充分记录,2018年新发结核病患者中有3.4%(95%CI 2.5%-4.4%)和先前接受治疗的结核病患者中有18%(95%CI 7.6%-31%)在全球和区域层面对异烟肼耐药的普遍性了解较少。2018年,世界卫生组织(WHO)建议对耐异烟肼,利福平易感性结核病(Hr-TB)的患者进行改良的6个月治疗方案,其中包括利福平,吡嗪酰胺,乙胺丁醇和左氧氟沙星。我们估计了结核病患者中Hr-TB的全球患病率,并调查了相关的表型和基因型耐药模式。方法和结果回顾了从2003年至2017年期间对结核病患者进行的常规连续监测或全国代表性的定期调查向世卫组织报告的总体耐药数据。从156个国家或地区可获得211,753名患者的异烟肼数据。其中,新发结核病患者的全球Hr-TB患病率为7.4%(95%CI 6.5%-8.4%),以前接受治疗的结核病患者中的Hr-TB患病率为11.4%(95%CI 9.4%-13.4%)。吡嗪酰胺和左氧氟沙星耐药性的其他数据可从6个国家(阿塞拜疆,孟加拉国,白俄罗斯,巴基斯坦,菲律宾和南非)获得。除菲律宾(1.8%,95%CI 0.2-6.4)和白俄罗斯(5.3%,95%CI 0.1-26.0)外,Hr-TB患者中均未出现对吡嗪酰胺和左氧氟沙星耐药的病例。有4,563例患者可获得有关异烟肼耐药性的所有基因组区域的测序数据。在通过表型测试或测序证实耐药的1,174株菌株中,有78.6%(95%CI 76.1%-80.9%)的katG基因具有耐药性突变,而二者均具有14.6%(95%CI 12.7%-16.8%)的耐药性。 katG和inhA启动子区域。对于6.8%(95%CI 5.4%-8.4%)的患者,仅在inhA启动子中发生突变,可以考虑增加异烟肼的剂量。这项研究的主要局限性在于,大多数分析是在国家一级而不是在单个患者一级进行的,并且实验室测试的质量在不同国家之间可能会有所不同。结论在这项研究中,结核病患者中Hr-TB的患病率高于全球对利福平耐药的患病率。利福平测试驱动的当前诊断算法会遗漏许多Hr-TB患者,这突出表明需要新的快速分子技术来确保获得适当的治疗和护理。Hr-TB患者对吡嗪酰胺和氟喹诺酮类药物的耐药性较低,为推荐的改良治疗方案提供了进一步的依据。全球结核病患者中Hr-TB的患病率高于利福平耐药性的患病率。利福平测试驱动的当前诊断算法会遗漏许多Hr-TB患者,这突出表明需要新的快速分子技术来确保获得适当的治疗和护理。Hr-TB患者对吡嗪酰胺和氟喹诺酮类药物的耐药性较低,为推荐的改良治疗方案提供了进一步的依据。全球结核病患者中Hr-TB的患病率高于利福平耐药性的患病率。利福平测试驱动的当前诊断算法会遗漏许多Hr-TB患者,这突出表明需要新的快速分子技术来确保获得适当的治疗和护理。Hr-TB患者对吡嗪酰胺和氟喹诺酮类药物的耐药性较低,为推荐的改良治疗方案提供了进一步的依据。
更新日期:2020-01-22
中文翻译:

结核患者异烟肼耐药性的流行和遗传学特征:横断面数据的多国分析。
背景技术在结核病(TB)患者中对耐药性的监测对于抵抗全球结核病流行和防止抗菌素耐药性的传播至关重要。异烟肼和利福平是最强大的一线抗结核药物中的两种,对它们中任何一种的抗药性都会增加治疗失败,复发或对其他药物产生抗药性的风险。利福平耐药性的全球患病率已得到充分记录,2018年新发结核病患者中有3.4%(95%CI 2.5%-4.4%)和先前接受治疗的结核病患者中有18%(95%CI 7.6%-31%)在全球和区域层面对异烟肼耐药的普遍性了解较少。2018年,世界卫生组织(WHO)建议对耐异烟肼,利福平易感性结核病(Hr-TB)的患者进行改良的6个月治疗方案,其中包括利福平,吡嗪酰胺,乙胺丁醇和左氧氟沙星。我们估计了结核病患者中Hr-TB的全球患病率,并调查了相关的表型和基因型耐药模式。方法和结果回顾了从2003年至2017年期间对结核病患者进行的常规连续监测或全国代表性的定期调查向世卫组织报告的总体耐药数据。从156个国家或地区可获得211,753名患者的异烟肼数据。其中,新发结核病患者的全球Hr-TB患病率为7.4%(95%CI 6.5%-8.4%),以前接受治疗的结核病患者中的Hr-TB患病率为11.4%(95%CI 9.4%-13.4%)。吡嗪酰胺和左氧氟沙星耐药性的其他数据可从6个国家(阿塞拜疆,孟加拉国,白俄罗斯,巴基斯坦,菲律宾和南非)获得。除菲律宾(1.8%,95%CI 0.2-6.4)和白俄罗斯(5.3%,95%CI 0.1-26.0)外,Hr-TB患者中均未出现对吡嗪酰胺和左氧氟沙星耐药的病例。有4,563例患者可获得有关异烟肼耐药性的所有基因组区域的测序数据。在通过表型测试或测序证实耐药的1,174株菌株中,有78.6%(95%CI 76.1%-80.9%)的katG基因具有耐药性突变,而二者均具有14.6%(95%CI 12.7%-16.8%)的耐药性。 katG和inhA启动子区域。对于6.8%(95%CI 5.4%-8.4%)的患者,仅在inhA启动子中发生突变,可以考虑增加异烟肼的剂量。这项研究的主要局限性在于,大多数分析是在国家一级而不是在单个患者一级进行的,并且实验室测试的质量在不同国家之间可能会有所不同。结论在这项研究中,结核病患者中Hr-TB的患病率高于全球对利福平耐药的患病率。利福平测试驱动的当前诊断算法会遗漏许多Hr-TB患者,这突出表明需要新的快速分子技术来确保获得适当的治疗和护理。Hr-TB患者对吡嗪酰胺和氟喹诺酮类药物的耐药性较低,为推荐的改良治疗方案提供了进一步的依据。全球结核病患者中Hr-TB的患病率高于利福平耐药性的患病率。利福平测试驱动的当前诊断算法会遗漏许多Hr-TB患者,这突出表明需要新的快速分子技术来确保获得适当的治疗和护理。Hr-TB患者对吡嗪酰胺和氟喹诺酮类药物的耐药性较低,为推荐的改良治疗方案提供了进一步的依据。全球结核病患者中Hr-TB的患病率高于利福平耐药性的患病率。利福平测试驱动的当前诊断算法会遗漏许多Hr-TB患者,这突出表明需要新的快速分子技术来确保获得适当的治疗和护理。Hr-TB患者对吡嗪酰胺和氟喹诺酮类药物的耐药性较低,为推荐的改良治疗方案提供了进一步的依据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号