当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Nitrogen-Carbon Electrocatalysts for CO2 Reduction Towards Syngas Generation.
ChemSusChem ( IF 7.5 ) Pub Date : 2020-01-21 , DOI: 10.1002/cssc.201903281 Laurent Delafontaine 1 , Tristan Asset 1 , Plamen Atanassov 1
ChemSusChem ( IF 7.5 ) Pub Date : 2020-01-21 , DOI: 10.1002/cssc.201903281 Laurent Delafontaine 1 , Tristan Asset 1 , Plamen Atanassov 1
Affiliation
|
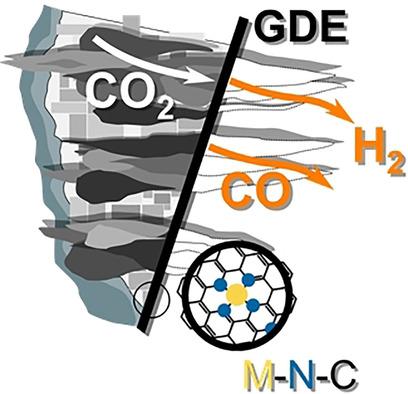
Shifting syngas (a H 2 :CO mix) production away from fossil-fuel dependent methods ( e.g. , steam methane reforming and coal gasification) is mandatory as syngas is of interest as both a fuel and as a value-added chemical precursor. When using the adequate electrocatalysts, such as silver-based or metal-nitrogen-carbon (M-N-C), the electrochemical CO 2 reduction reaction (CO 2 RR) allows for the production of CO alongside H 2 (from the hydrogen evolution reaction), thus leading to syngas generation. Here, we discuss the application of M-N-C electrocatalysts for syngas generation. The mechanisms leading to different faradaic selectivity for CO are reviewed as a function of the metallic element nature, using both computational and experimental approaches. The role played by the metal-free moieties in the metal-nitrogen-carbon electrocatalysts is underlined. Since the M-N-C electrocatalysts only recently entered the CO 2 RR field ( vs. the Cu, Ag or Au-based nanostructures), they have been mainly characterized in static liquid environments, where the reaction rate is significantly hampered by CO 2 -dissolution/diffusion limitations. Therefore, the design of CO 2 RR electrolyzers for M-N-C electrocatalysts is here addressed, highlighting designs such as zero-gap electrolyzers using anionic membranes and humidified CO 2 gas feed at the cathode.
中文翻译:

用于合成气生成的CO2还原的金属-氮-碳电催化剂。
必须将合成气(H 2:CO混合气)的生产从依赖化石燃料的方法(例如蒸汽甲烷重整和煤气化)中转移出来,因为合成气既有燃料用途,也有增值的化学前体,因此是必不可少的。当使用适当的电催化剂(例如银基或金属氮碳(MNC))时,电化学CO 2还原反应(CO 2 RR)可以与H 2一起生成CO(由析氢反应生成),因此导致合成气的产生。在这里,我们讨论了MNC电催化剂在合成气产生中的应用。使用计算和实验方法,对导致不同的法拉第选择性的CO的机理进行了审查,作为金属元素性质的函数。强调了无金属部分在金属-氮-碳电催化剂中的作用。由于MNC电催化剂仅在最近才进入CO 2 RR领域(相对于基于Cu,Ag或Au的纳米结构),它们的主要特征是在静态液体环境中,其中CO 2溶解/扩散显着阻碍了反应速率局限性。因此,这里讨论了用于MNC电催化剂的CO 2 RR电解槽的设计,重点介绍了诸如采用阴离子膜和在阴极加湿的CO 2气体进料的零间隙电解槽等设计。反应速率受到CO 2溶解/扩散限制的严重阻碍。因此,这里讨论了用于MNC电催化剂的CO 2 RR电解槽的设计,重点介绍了诸如采用阴离子膜和在阴极加湿的CO 2气体进料的零间隙电解槽等设计。反应速率受到CO 2溶解/扩散限制的严重阻碍。因此,这里讨论了用于MNC电催化剂的CO 2 RR电解槽的设计,重点介绍了诸如采用阴离子膜和在阴极加湿的CO 2气体进料的零间隙电解槽等设计。
更新日期:2020-03-19
中文翻译:

用于合成气生成的CO2还原的金属-氮-碳电催化剂。
必须将合成气(H 2:CO混合气)的生产从依赖化石燃料的方法(例如蒸汽甲烷重整和煤气化)中转移出来,因为合成气既有燃料用途,也有增值的化学前体,因此是必不可少的。当使用适当的电催化剂(例如银基或金属氮碳(MNC))时,电化学CO 2还原反应(CO 2 RR)可以与H 2一起生成CO(由析氢反应生成),因此导致合成气的产生。在这里,我们讨论了MNC电催化剂在合成气产生中的应用。使用计算和实验方法,对导致不同的法拉第选择性的CO的机理进行了审查,作为金属元素性质的函数。强调了无金属部分在金属-氮-碳电催化剂中的作用。由于MNC电催化剂仅在最近才进入CO 2 RR领域(相对于基于Cu,Ag或Au的纳米结构),它们的主要特征是在静态液体环境中,其中CO 2溶解/扩散显着阻碍了反应速率局限性。因此,这里讨论了用于MNC电催化剂的CO 2 RR电解槽的设计,重点介绍了诸如采用阴离子膜和在阴极加湿的CO 2气体进料的零间隙电解槽等设计。反应速率受到CO 2溶解/扩散限制的严重阻碍。因此,这里讨论了用于MNC电催化剂的CO 2 RR电解槽的设计,重点介绍了诸如采用阴离子膜和在阴极加湿的CO 2气体进料的零间隙电解槽等设计。反应速率受到CO 2溶解/扩散限制的严重阻碍。因此,这里讨论了用于MNC电催化剂的CO 2 RR电解槽的设计,重点介绍了诸如采用阴离子膜和在阴极加湿的CO 2气体进料的零间隙电解槽等设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号