当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of 1-alkyl-5/6-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)-1H-indole-3-carbonitriles as novel xanthine oxidase inhibitors.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.ejmech.2020.112077 Jun Gao 1 , Xuegui Liu 2 , Bing Zhang 1 , Qing Mao 1 , Zhuo Zhang 1 , Qian Zou 3 , Xiwen Dai 1 , Shaojie Wang 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.ejmech.2020.112077 Jun Gao 1 , Xuegui Liu 2 , Bing Zhang 1 , Qing Mao 1 , Zhuo Zhang 1 , Qian Zou 3 , Xiwen Dai 1 , Shaojie Wang 1
Affiliation
|
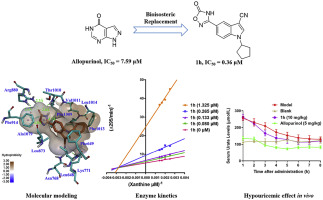
Xanthine oxidase (XO) has emerged as an important target for the treatment of hyperuricemia and gout. In this study, to obtain novel nonpurine XO inhibitors, a series of 1-alkyl-5/6-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)-1H-indole-3-carbonitriles (1a-1u, 2c, 2e, 2h and 2n) were designed using a bioisosteric replacement strategy and were synthesized through a five-step procedure with good yields. Thereafter, the in vitro XO inhibitory potencies of these compounds were evaluated by spectrophotometry, showing inhibitory profiles in the micromolar/submicromolar range. Particularly, compound 1h emerged as the strongest XO inhibitor, with an IC50 value of 0.36 μM, which was approximately 21-fold more potent than the positive control allopurinol. Additionally, the structure-activity relationships revealed that the 5-oxo-4,5-dihydro-1,2,4-oxadiazole moiety linked at the 5-position of the indole scaffold was more preferable than the 6-position for the XO inhibitory potency. Enzyme kinetic studies indicated that compound 1h acted as a mixed-type XO inhibitor. Moreover, molecular modeling studies were performed on compound 1h to gain insights into its binding modes with XO. The results showed that the 5-oxo-4,5-dihydro-1,2,4-oxadiazole moiety could interact with Arg880 and Thr1010 in the innermost part of the active pocket through hydrogen bonds, while the cyano group could form hydrogen bonds with Asn768 and Lys771 in the subpocket. Furthermore, the in vivo hypouricemic effect of compound 1h was further investigated in a hyperuricemia rat model induced by potassium oxonate. The results suggested that compound 1h could effectively reduce serum uric acid levels at an oral dose of 10 mg/kg. Therefore, compound 1h could be a promising lead compound for the treatment of hyperuricemia and gout.
中文翻译:

作为新的黄嘌呤氧化酶的1-烷基-5 / 6-(5-氧代-4,5-二氢-1,2,4-恶二唑-3-基)-1H-吲哚-3-腈的设计,合成及生物学评价抑制剂。
黄嘌呤氧化酶(XO)已成为治疗高尿酸血症和痛风的重要靶标。在这项研究中,要获得新型的非嘌呤XO抑制剂,需要一系列1-烷基-5 / 6-(5-氧代-4,5-二氢-1,2,4-氧二唑-3-基)-1H-吲哚-使用生物等位取代策略设计3-腈(1a-1u,2c,2e,2h和2n),并通过五步法合成,收率高。此后,通过分光光度法评估了这些化合物的体外XO抑制能力,显示了在微摩尔/亚微摩尔范围内的抑制特性。特别地,化合物1h成为最强的XO抑制剂,IC50值为0.36μM,比阳性对照别嘌呤醇的效价高约21倍。此外,结构与活性的关系揭示了5-oxo-4,5-dihydro-1,2,在XO抑制效能上,在吲哚支架的5-位连接的4-恶二唑部分比6-位更优选。酶动力学研究表明,化合物1h充当混合型XO抑制剂。此外,对化合物1h进行了分子建模研究,以深入了解其与XO的结合模式。结果表明,5-oxo-4,5-dihydro-1,2,4-oxadiazole部分可通过氢键与活性囊最内部的Arg880和Thr1010相互作用,而氰基可与氢键形成氢键。子口袋中的Asn768和Lys771。此外,在由草酸钾诱导的高尿酸血症大鼠模型中进一步研究了化合物1h的体内降尿酸作用。结果表明,口服剂量为10 mg / kg时,化合物1h可以有效降低血清尿酸水平。因此,化合物1h可能是治疗高尿酸血症和痛风的有前途的先导化合物。
更新日期:2020-01-22
中文翻译:

作为新的黄嘌呤氧化酶的1-烷基-5 / 6-(5-氧代-4,5-二氢-1,2,4-恶二唑-3-基)-1H-吲哚-3-腈的设计,合成及生物学评价抑制剂。
黄嘌呤氧化酶(XO)已成为治疗高尿酸血症和痛风的重要靶标。在这项研究中,要获得新型的非嘌呤XO抑制剂,需要一系列1-烷基-5 / 6-(5-氧代-4,5-二氢-1,2,4-氧二唑-3-基)-1H-吲哚-使用生物等位取代策略设计3-腈(1a-1u,2c,2e,2h和2n),并通过五步法合成,收率高。此后,通过分光光度法评估了这些化合物的体外XO抑制能力,显示了在微摩尔/亚微摩尔范围内的抑制特性。特别地,化合物1h成为最强的XO抑制剂,IC50值为0.36μM,比阳性对照别嘌呤醇的效价高约21倍。此外,结构与活性的关系揭示了5-oxo-4,5-dihydro-1,2,在XO抑制效能上,在吲哚支架的5-位连接的4-恶二唑部分比6-位更优选。酶动力学研究表明,化合物1h充当混合型XO抑制剂。此外,对化合物1h进行了分子建模研究,以深入了解其与XO的结合模式。结果表明,5-oxo-4,5-dihydro-1,2,4-oxadiazole部分可通过氢键与活性囊最内部的Arg880和Thr1010相互作用,而氰基可与氢键形成氢键。子口袋中的Asn768和Lys771。此外,在由草酸钾诱导的高尿酸血症大鼠模型中进一步研究了化合物1h的体内降尿酸作用。结果表明,口服剂量为10 mg / kg时,化合物1h可以有效降低血清尿酸水平。因此,化合物1h可能是治疗高尿酸血症和痛风的有前途的先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号