当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dynamics and Location of the Allosteric Midazolam Site in Cytochrome P4503A4 in Lipid Nanodiscs.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.biochem.9b01001 Michelle Redhair 1 , John C Hackett 2 , Robert D Pelletier 1 , William M Atkins 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.biochem.9b01001 Michelle Redhair 1 , John C Hackett 2 , Robert D Pelletier 1 , William M Atkins 1
Affiliation
|
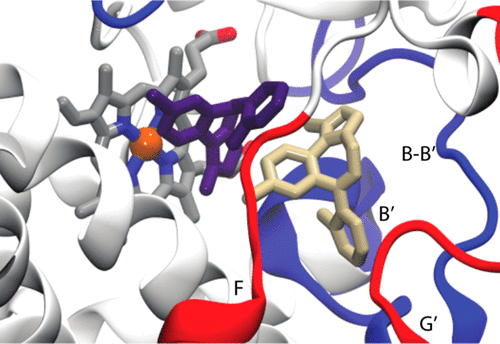
Promiscuous and allosteric drug interactions with cytochrome P450 3A4 (CYP3A4) are ubiquitous but incompletely understood at the molecular level. A classic allosteric CYP3A4 drug interaction includes the benzodiazepine midazolam (MDZ). MDZ exhibits homotropic and heterotropic allostery when metabolized to 1'-hydroxy and 4-hydroxy metabolites in varying ratios. The combination of hydrogen-deuterium exchange mass spectrometry (HDX-MS) and Gaussian accelerated molecular dynamics (GaMD) simulations of CYP3A4 in lipid nanodiscs and in a lipid bilayer, respectively, reveals MDZ-dependent changes in dynamics in a membrane environment. The F-, G-, and intervening helices, as well as the loop preceding the β1-sheets, display the largest observed changes in HDX. The GaMD suggests a potential allosteric binding site for MDZ in the F'- and G'-regions, which undergo significant increases in HDX at near-saturating MDZ concentrations. The HDX-MS and GaMD results confirm that changes in dynamics are most significant near the developing consensus allosteric site, and these changes are distinct from those observed previously with the nonallosteric inhibitor ketoconazole. The results suggest that the allosteric MDZ remains mobile in its binding site at the Phe-cluster. The results further suggest that this binding site remains dynamic or changes the depth of insertion in the membrane.
中文翻译:

脂质纳米盘中细胞色素P4503A4的变构咪达唑仑位点的动力学和位置。
与细胞色素P450 3A4(CYP3A4)的混杂和变构药物相互作用是普遍存在的,但在分子水平上尚不完全清楚。CYP3A4的经典变构药物相互作用包括苯并二氮杂咪达唑仑(MDZ)。当以不同比例代谢成1'-羟基和4-羟基代谢物时,MDZ表现出同质和异质变构。氢-氘交换质谱(HDX-MS)和高斯加速分子动力学(GaMD)对CYP3A4在脂质纳米盘和脂质双层中的模拟的结合,分别揭示了膜环境中MDZ依赖的动力学变化。F螺旋,G螺旋和中间螺旋以及β1折叠之前的环在HDX中显示出最大的变化。GaMD表明在F'-和G'中可能存在MDZ的变构结合位点 -区域,在接近饱和的MDZ浓度下HDX显着增加。HDX-MS和GaMD结果证实,动力学变化在正在形成的共有变构位点附近最为显着,并且这些变化与之前使用非变构抑制剂酮康唑观察到的变化不同。结果表明,变构MDZ在Phe簇的结合位点保持可移动。结果进一步表明该结合位点保持动态或改变了在膜中的插入深度。这些变化与以前使用非变构抑制剂酮康唑观察到的变化不同。结果表明,变构MDZ在Phe簇的结合位点保持可移动。结果进一步表明该结合位点保持动态或改变膜中插入的深度。这些变化与以前使用非变构抑制剂酮康唑观察到的变化不同。结果表明,变构MDZ在Phe簇的结合位点保持可移动。结果进一步表明该结合位点保持动态或改变膜中插入的深度。
更新日期:2020-01-27
中文翻译:

脂质纳米盘中细胞色素P4503A4的变构咪达唑仑位点的动力学和位置。
与细胞色素P450 3A4(CYP3A4)的混杂和变构药物相互作用是普遍存在的,但在分子水平上尚不完全清楚。CYP3A4的经典变构药物相互作用包括苯并二氮杂咪达唑仑(MDZ)。当以不同比例代谢成1'-羟基和4-羟基代谢物时,MDZ表现出同质和异质变构。氢-氘交换质谱(HDX-MS)和高斯加速分子动力学(GaMD)对CYP3A4在脂质纳米盘和脂质双层中的模拟的结合,分别揭示了膜环境中MDZ依赖的动力学变化。F螺旋,G螺旋和中间螺旋以及β1折叠之前的环在HDX中显示出最大的变化。GaMD表明在F'-和G'中可能存在MDZ的变构结合位点 -区域,在接近饱和的MDZ浓度下HDX显着增加。HDX-MS和GaMD结果证实,动力学变化在正在形成的共有变构位点附近最为显着,并且这些变化与之前使用非变构抑制剂酮康唑观察到的变化不同。结果表明,变构MDZ在Phe簇的结合位点保持可移动。结果进一步表明该结合位点保持动态或改变了在膜中的插入深度。这些变化与以前使用非变构抑制剂酮康唑观察到的变化不同。结果表明,变构MDZ在Phe簇的结合位点保持可移动。结果进一步表明该结合位点保持动态或改变膜中插入的深度。这些变化与以前使用非变构抑制剂酮康唑观察到的变化不同。结果表明,变构MDZ在Phe簇的结合位点保持可移动。结果进一步表明该结合位点保持动态或改变膜中插入的深度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号