当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Annealing in the Close Vicinity of Tg on the Reorganization within Dimers and Its Impact on the Crystallization Kinetics of Gemfibrozil.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.molpharmaceut.9b01244 Ewa Kamińska 1 , Aldona Minecka 1 , Magdalena Tarnacka 2, 3 , Barbara Hachuła 4 , Kamil Kamiński 2, 3 , Marian Paluch 2, 3
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.molpharmaceut.9b01244 Ewa Kamińska 1 , Aldona Minecka 1 , Magdalena Tarnacka 2, 3 , Barbara Hachuła 4 , Kamil Kamiński 2, 3 , Marian Paluch 2, 3
Affiliation
|
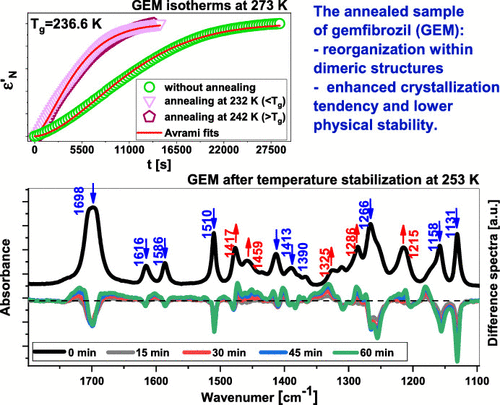
In this paper, broadband dielectric spectroscopy (BDS) has been applied to study the molecular dynamics and crystallization kinetics of the antihyperlipidemic active pharmaceutical ingredient (API), gemfibrozil (GEM), as well as its deuterated (dGEM) and methylated (metGEM) derivatives, characterized by different types and strengths of intermolecular interactions. Moreover, calorimetric and infrared measurements have been carried out to characterize the thermal properties of examined samples and to probe a change in the H-bonding pattern in GEM, respectively. We found that the dielectric spectra of all examined compounds, collected below the glass transition temperature (Tg), reveal the presence of two secondary relaxations (β, γ). According to the coupling model (CM) predictions, it was assumed that the slower process (β) is of JG type, whereas the faster one (γ) has an intramolecular origin. Interestingly, the extensive crystallization kinetics measurements performed after applying two paths, i.e., the standard procedure (cooling and subsequently heating up to the appropriate temperature, Tc), as well as annealing at two temperatures in the vicinity of Tg and further heating up to Tc, showed that the annealing increases the crystallization rate in the case of native API, while the thermal history of the sample has no significant impact on the pace of this process in the two derivatives of GEM. Analysis of the dielectric strength (Δε) of the α-process during annealing, together with the results of Fourier transform infrared spectroscopy (FTIR) measurements, suggested that the reorganization within dimeric structures formed between the GEM molecules is responsible for the observed behavior. Importantly, our results differ from those obtained by Tominaka et al. (Tominaka, S.; Kawakami, K.; Fukushima, M.; Miyazaki, A.Physical Stabilization of Pharmaceutical Glasses Based on Hydrogen Bond Reorganization under Sub-Tg Temperature Mol. Pharm. 2017 14 264 273 10.1021/acs.molpharmaceut.6b00866.), who demonstrated that the sub-Tg annealing of ritonavir (RTV), which is able to form extensive supramolecular hydrogen bonds, protects this active substance against crystallization. Therefore, based on these contradictory reports, one can hypothesize that materials forming H-bonded structures, characterized by varying architecture, may behave differently after annealing in the vicinity of the glass transition temperature.
中文翻译:

Tg的近邻退火对二聚体内部重组的影响及其对吉非贝齐结晶动力学的影响。
在本文中,宽带介电谱(BDS)已用于研究抗高血脂活性药物成分(API),吉非贝齐(GEM)以及其氘代(dGEM)和甲基化(metGEM)衍生物的分子动力学和结晶动力学,其特点是分子间相互作用的类型和强度不同。此外,已经进行了量热法和红外测量,以表征所检查样品的热性质,并探查GEM中H键模式的变化。我们发现在玻璃化转变温度(Tg)以下收集的所有受检化合物的介电谱显示存在两个次级弛豫(β,γ)。根据耦合模型(CM)的预测,假设较慢的过程(β)为JG类型,而速度更快的(γ)具有分子内起源。有趣的是,广泛的结晶动力学测量是在应用两条路径后进行的,即标准程序(冷却并随后加热至合适的温度Tc),以及在Tg附近的两个温度下退火并进一步加热至Tc的结果表明,在天然原料药的情况下,退火提高了结晶速率,而样品的热历史对GEM的两种衍生物中的这一过程的速度没有显着影响。分析退火过程中α过程的介电强度(Δε),以及傅立叶变换红外光谱(FTIR)测量结果,提示在GEM分子之间形成的二聚体结构内的重组是所观察到的行为的原因。重要的是,我们的结果不同于Tominaka等人获得的结果。(Tominaka,S .; Kawakami,K .; Fukushima,M .; Miyazaki,A.基于低于Tg温度的氢键重组的药物玻璃的物理稳定性Mol.Pharm.2017 14 264 273 10.1021 / acs.molpharmaceut.6b00866 ·················································································································································································· 因此,根据这些相互矛盾的报告,我们可以假设形成H键结构的材料具有不同的结构,
更新日期:2020-02-06
中文翻译:

Tg的近邻退火对二聚体内部重组的影响及其对吉非贝齐结晶动力学的影响。
在本文中,宽带介电谱(BDS)已用于研究抗高血脂活性药物成分(API),吉非贝齐(GEM)以及其氘代(dGEM)和甲基化(metGEM)衍生物的分子动力学和结晶动力学,其特点是分子间相互作用的类型和强度不同。此外,已经进行了量热法和红外测量,以表征所检查样品的热性质,并探查GEM中H键模式的变化。我们发现在玻璃化转变温度(Tg)以下收集的所有受检化合物的介电谱显示存在两个次级弛豫(β,γ)。根据耦合模型(CM)的预测,假设较慢的过程(β)为JG类型,而速度更快的(γ)具有分子内起源。有趣的是,广泛的结晶动力学测量是在应用两条路径后进行的,即标准程序(冷却并随后加热至合适的温度Tc),以及在Tg附近的两个温度下退火并进一步加热至Tc的结果表明,在天然原料药的情况下,退火提高了结晶速率,而样品的热历史对GEM的两种衍生物中的这一过程的速度没有显着影响。分析退火过程中α过程的介电强度(Δε),以及傅立叶变换红外光谱(FTIR)测量结果,提示在GEM分子之间形成的二聚体结构内的重组是所观察到的行为的原因。重要的是,我们的结果不同于Tominaka等人获得的结果。(Tominaka,S .; Kawakami,K .; Fukushima,M .; Miyazaki,A.基于低于Tg温度的氢键重组的药物玻璃的物理稳定性Mol.Pharm.2017 14 264 273 10.1021 / acs.molpharmaceut.6b00866 ·················································································································································································· 因此,根据这些相互矛盾的报告,我们可以假设形成H键结构的材料具有不同的结构,











































 京公网安备 11010802027423号
京公网安备 11010802027423号