当前位置:
X-MOL 学术
›
ChemPhotoChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calcium Bismuthate (CaBiO3): A Potential Sunlight‐Driven Perovskite Photocatalyst for the Degradation of Emerging Pharmaceutical Contaminants
ChemPhotoChem ( IF 3.0 ) Pub Date : 2020-01-27 , DOI: 10.1002/cptc.201900265 Karuppannan Rokesh 1 , Mohan Sakar 1, 2 , Trong‐On Do 1
ChemPhotoChem ( IF 3.0 ) Pub Date : 2020-01-27 , DOI: 10.1002/cptc.201900265 Karuppannan Rokesh 1 , Mohan Sakar 1, 2 , Trong‐On Do 1
Affiliation
|
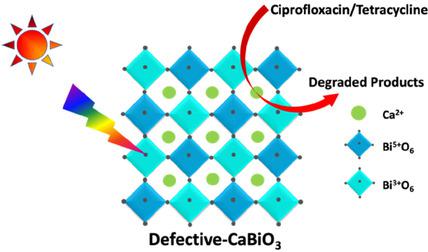
This study reports the photocatalytic efficiencies of CaBiO3 (CBO) towards the degradation of contaminants of emerging concern (CEC), such as ciprofloxacin and tetracycline, under solar light irradiation. CaBiO3 was synthesized using glycine‐complexation (GC) and ion‐exchange (IE) methods. The structural difference of materials obtained by these different methods was evidenced from XRD and XPS results, and were due to the induced dual oxidation state of Bi (Bi3+/5+) in CaBiO3. The CBO synthesized by the GC method (CBO‐GC) showed a broad UV/Vis absorption range from 200 to 550 nm and a band gap energy of 2.30 eV, while it was found to be 200 to 450 nm and 2.65 eV for the CBO synthesized by the IE method (CBO‐IE). The VB potential of CBO‐IE is shifted towards more positive potentials (+2.45 eV) as compared to the CBO‐GC (+1.90 eV), and thereby the calculated CB potential of CBO‐GC and CBO‐IE was found to be −0.40 and −0.20 eV, respectively. Such positioning of the VB and CB favored the generation of highly reactive radicals towards the effective degradation of pollutants. These relative alignments of the bands could be mediated by the oxygen vacancy defects in the systems owing to the dual oxidation state of Bi. The photocurrent and impedance measurements demonstrated excellent charge separation and lower resistivity in CBO‐GC as compared to the CBO‐IE. Accordingly, the CBO‐GC showed enhanced photocatalytic degradation efficiency, improved photostability and reusability as compared to that of the CBO‐IE.
中文翻译:

铋酸钙(CaBiO3):潜在的阳光驱动钙钛矿光催化剂,用于降解新兴的药用污染物。
这项研究报告了在太阳光照射下,CaBiO 3(CBO)对新出现的污染物(CEC)的降解的光催化效率,如环丙沙星和四环素。CaBiO 3使用甘氨酸络合(GC)和离子交换(IE)方法合成。通过XRD和XPS结果证明了通过这些不同方法获得的材料的结构差异,这是由于Bi(Bi 3 + / 5 +)在CaBiO 3中的诱导双氧化态引起的。。通过GC方法合成的CBO(CBO-GC)在200至550 nm范围内具有宽的UV / Vis吸收范围和2.30 eV的带隙能量,而CBO的CBO在200至450 nm和2.65 eV的范围内通过IE方法(CBO‐IE)合成。与CBO-GC(+1.90 eV)相比,CBO-IE的VB电位朝着更正的电位(+2.45 eV)移动,因此发现计算出的CBO-GC和CBO-IE的CB电位为-分别为0.40和-0.20 eV。VB和CB的这种定位有利于产生高反应性的自由基,从而有效地降解污染物。由于Bi的双重氧化态,这些能带的相对排列可以由系统中的氧空位缺陷来介导。与CBO-IE相比,光电流和阻抗测量显示出CBO-GC中出色的电荷分离和较低的电阻率。因此,与CBO-IE相比,CBO-GC显示出更高的光催化降解效率,更高的光稳定性和可重复使用性。
更新日期:2020-01-27
中文翻译:

铋酸钙(CaBiO3):潜在的阳光驱动钙钛矿光催化剂,用于降解新兴的药用污染物。
这项研究报告了在太阳光照射下,CaBiO 3(CBO)对新出现的污染物(CEC)的降解的光催化效率,如环丙沙星和四环素。CaBiO 3使用甘氨酸络合(GC)和离子交换(IE)方法合成。通过XRD和XPS结果证明了通过这些不同方法获得的材料的结构差异,这是由于Bi(Bi 3 + / 5 +)在CaBiO 3中的诱导双氧化态引起的。。通过GC方法合成的CBO(CBO-GC)在200至550 nm范围内具有宽的UV / Vis吸收范围和2.30 eV的带隙能量,而CBO的CBO在200至450 nm和2.65 eV的范围内通过IE方法(CBO‐IE)合成。与CBO-GC(+1.90 eV)相比,CBO-IE的VB电位朝着更正的电位(+2.45 eV)移动,因此发现计算出的CBO-GC和CBO-IE的CB电位为-分别为0.40和-0.20 eV。VB和CB的这种定位有利于产生高反应性的自由基,从而有效地降解污染物。由于Bi的双重氧化态,这些能带的相对排列可以由系统中的氧空位缺陷来介导。与CBO-IE相比,光电流和阻抗测量显示出CBO-GC中出色的电荷分离和较低的电阻率。因此,与CBO-IE相比,CBO-GC显示出更高的光催化降解效率,更高的光稳定性和可重复使用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号