当前位置:
X-MOL 学术
›
ChemPhotoChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Synthetic Strategy for the Structural Modification of Photoactivatable BODIPY‐Oxazine Dyads
ChemPhotoChem ( IF 3.0 ) Pub Date : 2020-02-03 , DOI: 10.1002/cptc.201900276 Ek Raj Thapaliya 1 , Mercedes M. A. Mazza 1 , Janet Cusido 1, 2 , James D. Baker 1 , Françisco M. Raymo 1
ChemPhotoChem ( IF 3.0 ) Pub Date : 2020-02-03 , DOI: 10.1002/cptc.201900276 Ek Raj Thapaliya 1 , Mercedes M. A. Mazza 1 , Janet Cusido 1, 2 , James D. Baker 1 , Françisco M. Raymo 1
Affiliation
|
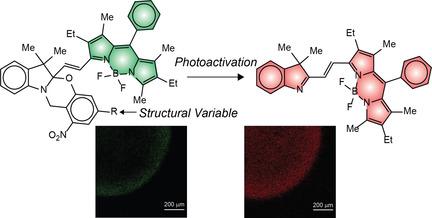
Strategies to regulate the ability of photoactivatable fluorophores to absorb activating photons are essential to allow their operation under illumination conditions compatible with biological samples. In this context, our laboratories identified a viable synthetic protocol to introduce a conjugated substituent on the photocleavable component of a BODIPY‐oxazine dyad and enhance its molar absorption coefficient at the activation wavelength. This structural transformation translates into a four‐fold absorbance increase in the spectral window appropriate for photoactivation and does not prevent the photochemical transformation. Indeed, the photoinduced cleavage of the oxazine component occurs in organic solvents, aqueous solutions and hydrogel matrices with a concomitant bathochromic shift in BODIPY emission. These results demonstrate that the photophysical properties of this family of BODIPY‐oxazine dyads can be manipulated with the aid of chemical synthesis without suppressing their photochemical behavior.
中文翻译:

光活化BODIPY-恶嗪二元化合物结构修饰的合成策略
调节可光活化荧光团吸收活化光子能力的策略对于使其在与生物样品相容的光照条件下运行至关重要。在这种情况下,我们的实验室确定了一种可行的合成方案,可以将共轭取代基引入BODIPY-恶嗪二元组的光可裂解组分上,并提高其在激活波长下的摩尔吸收系数。这种结构转变会在适合光激活的光谱窗口中将吸收率提高四倍,并且不会阻止光化学转变。实际上,恶嗪组分的光诱导裂解发生在有机溶剂,水溶液和水凝胶基质中,并伴有BODIPY发射的红移。
更新日期:2020-02-03
中文翻译:

光活化BODIPY-恶嗪二元化合物结构修饰的合成策略
调节可光活化荧光团吸收活化光子能力的策略对于使其在与生物样品相容的光照条件下运行至关重要。在这种情况下,我们的实验室确定了一种可行的合成方案,可以将共轭取代基引入BODIPY-恶嗪二元组的光可裂解组分上,并提高其在激活波长下的摩尔吸收系数。这种结构转变会在适合光激活的光谱窗口中将吸收率提高四倍,并且不会阻止光化学转变。实际上,恶嗪组分的光诱导裂解发生在有机溶剂,水溶液和水凝胶基质中,并伴有BODIPY发射的红移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号