当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrafast Reaction Mechanisms in Perovskite Based Photocatalytic C–C Coupling
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-01-27 , DOI: 10.1021/acsenergylett.9b02714 Kang Wang 1, 2 , Haipeng Lu 2 , Xiaolin Zhu 3 , Yixiong Lin 3 , Matthew C. Beard 2 , Yong Yan 3 , Xihan Chen 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-01-27 , DOI: 10.1021/acsenergylett.9b02714 Kang Wang 1, 2 , Haipeng Lu 2 , Xiaolin Zhu 3 , Yixiong Lin 3 , Matthew C. Beard 2 , Yong Yan 3 , Xihan Chen 2
Affiliation
|
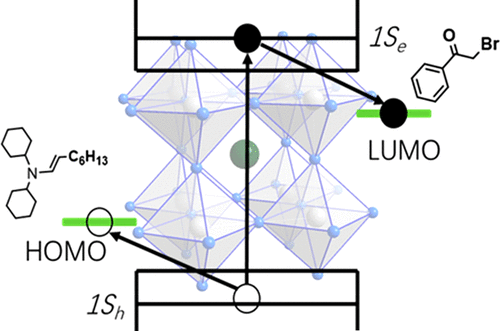
Solar driven carbon–carbon (C–C) bond formation is a new direction in solar energy utilization. Earth abundant nanocrystal based photocatalysts are highly sought after as they can potentially eliminate expensive noble metal catalysts. A detailed understanding of the underlying reaction mechanisms could provide guidance in designing new systems that can activate a larger class of small molecules. Here, we employ transient absorption spectroscopy to study a model C–C bond formation reaction, i.e., α-alkylation of aldehydes catalyzed by colloidal CsPbBr3 nanocrystals (NCs). We find that both electrons and holes undergo ultrafast charge transfer (∼50 ps) from photoexcited perovskite NCs to reactant molecules. A charge separated state lives for more than 0.8 μs, enabling a radical mechanism to form the C–C bonds. We discuss the differences between the NCs photoredox catalysts and the molecular catalyst.
中文翻译:

钙钛矿基光催化CC偶联中的超快反应机理
太阳能驱动的碳-碳(CC)键的形成是太阳能利用的新方向。富含地球的纳米晶体基光催化剂备受追捧,因为它们可以潜在地消除昂贵的贵金属催化剂。对潜在反应机制的详细了解可以为设计新系统提供指导,该系统可以激活更大种类的小分子。在这里,我们使用瞬态吸收光谱研究模型C–C键形成反应,即胶体CsPbBr 3催化醛的α-烷基化纳米晶体(NCs)。我们发现电子和空穴都经历了从光激发钙钛矿NCs到反应物分子的超快速电荷转移(〜50 ps)。电荷分离态的寿命超过0.8μs,从而使自由基机理形成了C–C键。我们讨论了NCs光氧化还原催化剂和分子催化剂之间的差异。
更新日期:2020-01-27
中文翻译:

钙钛矿基光催化CC偶联中的超快反应机理
太阳能驱动的碳-碳(CC)键的形成是太阳能利用的新方向。富含地球的纳米晶体基光催化剂备受追捧,因为它们可以潜在地消除昂贵的贵金属催化剂。对潜在反应机制的详细了解可以为设计新系统提供指导,该系统可以激活更大种类的小分子。在这里,我们使用瞬态吸收光谱研究模型C–C键形成反应,即胶体CsPbBr 3催化醛的α-烷基化纳米晶体(NCs)。我们发现电子和空穴都经历了从光激发钙钛矿NCs到反应物分子的超快速电荷转移(〜50 ps)。电荷分离态的寿命超过0.8μs,从而使自由基机理形成了C–C键。我们讨论了NCs光氧化还原催化剂和分子催化剂之间的差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号