当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of a 2,3-Benzodiazepine Intermediate of BET Inhibitor BAY 1238097 via Catalytic Asymmetric Hydrogenation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.oprd.9b00519 Gerard K. M. Verzijl 1 , Jorma Hassfeld 2 , André H. M. de Vries 1 , Laurent Lefort 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.oprd.9b00519 Gerard K. M. Verzijl 1 , Jorma Hassfeld 2 , André H. M. de Vries 1 , Laurent Lefort 1
Affiliation
|
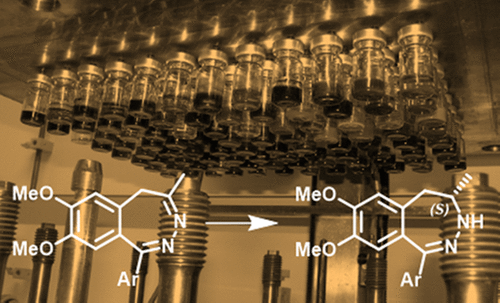
Herein, we report the first example of the asymmetric hydrogenation of a prochiral benzodiazepine substrate as key transformation in a pilot-scale synthesis of BET inhibitor BAY 1238097. High-throughput screening in a parallel reactor enabled us to identify an efficient catalyst based on Ir and a chiral bisphosphine. An additive screening allowed significant reduction of catalyst loading. Ultimately, the hydrogenation was performed on a kilogram scale leading to the production of 27 kg of the desired product with an enantiomeric excess of 99% after crystallization.
中文翻译:

通过催化不对称加氢对映选择性合成BET抑制剂BAY 1238097的2,3-苯二氮卓中间体
在此,我们报道了前手性苯并二氮杂底物的不对称氢化作为BET抑制剂BAY 1238097的中试规模合成中的关键转化的第一个例子。在并行反应器中的高通量筛选使我们能够鉴定基于Ir和Ib的有效催化剂手性双膦。添加剂筛选可以显着降低催化剂的负载量。最终,以千克规模进行氢化,从而在结晶后产生27kg的所需产物,对映体过量为99%。
更新日期:2020-02-06
中文翻译:

通过催化不对称加氢对映选择性合成BET抑制剂BAY 1238097的2,3-苯二氮卓中间体
在此,我们报道了前手性苯并二氮杂底物的不对称氢化作为BET抑制剂BAY 1238097的中试规模合成中的关键转化的第一个例子。在并行反应器中的高通量筛选使我们能够鉴定基于Ir和Ib的有效催化剂手性双膦。添加剂筛选可以显着降低催化剂的负载量。最终,以千克规模进行氢化,从而在结晶后产生27kg的所需产物,对映体过量为99%。










































 京公网安备 11010802027423号
京公网安备 11010802027423号