Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients
JAMA ( IF 63.1 ) Pub Date : 2020-01-21 , DOI: 10.1001/jama.2019.20747 Murali K Matta 1 , Jeffry Florian 1 , Robbert Zusterzeel 1 , Nageswara R Pilli 1 , Vikram Patel 1 , Donna A Volpe 1 , Yang Yang 2 , Luke Oh 3 , Edward Bashaw 3 , Issam Zineh 3 , Carlos Sanabria 4 , Sarah Kemp 4 , Anthony Godfrey 4 , Steven Adah 5 , Sergio Coelho 5 , Jian Wang 6 , Lesley-Anne Furlong 6 , Charles Ganley 6 , Theresa Michele 5 , David G Strauss 1
JAMA ( IF 63.1 ) Pub Date : 2020-01-21 , DOI: 10.1001/jama.2019.20747 Murali K Matta 1 , Jeffry Florian 1 , Robbert Zusterzeel 1 , Nageswara R Pilli 1 , Vikram Patel 1 , Donna A Volpe 1 , Yang Yang 2 , Luke Oh 3 , Edward Bashaw 3 , Issam Zineh 3 , Carlos Sanabria 4 , Sarah Kemp 4 , Anthony Godfrey 4 , Steven Adah 5 , Sergio Coelho 5 , Jian Wang 6 , Lesley-Anne Furlong 6 , Charles Ganley 6 , Theresa Michele 5 , David G Strauss 1
Affiliation
|
Importance
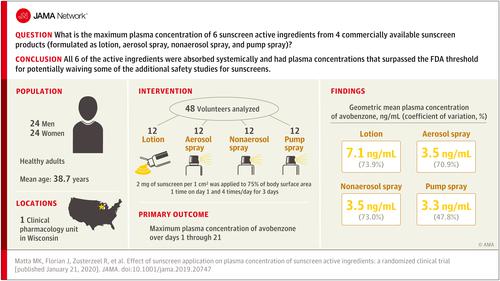
A prior pilot study demonstrated the systemic absorption of 4 sunscreen active ingredients; additional studies are needed to determine the systemic absorption of additional active ingredients and how quickly systemic exposure exceeds 0.5 ng/mL as recommended by the US Food and Drug Administration (FDA). Objective
To assess the systemic absorption and pharmacokinetics of the 6 active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate) in 4 sunscreen products under single- and maximal-use conditions. Design, Setting, and Participants
Randomized clinical trial at a clinical pharmacology unit (West Bend, Wisconsin) was conducted in 48 healthy participants. The study was conducted between January and February 2019. Interventions
Participants were randomized to 1 of 4 sunscreen products, formulated as lotion (n = 12), aerosol spray (n = 12), nonaerosol spray (n = 12), and pump spray (n = 12). Sunscreen product was applied at 2 mg/cm2 to 75% of body surface area at 0 hours on day 1 and 4 times on day 2 through day 4 at 2-hour intervals, and 34 blood samples were collected over 21 days from each participant. Main Outcomes and Measures
The primary outcome was the maximum plasma concentration of avobenzone over days 1 through 21. Secondary outcomes were the maximum plasma concentrations of oxybenzone, octocrylene, homosalate, octisalate, and octinoxate over days 1 through 21. Results
Among 48 randomized participants (mean [SD] age, 38.7 [13.2] years; 24 women [50%]; 23 white [48%], 23 African American [48%], 1 Asian [2%], and 1 of unknown race/ethnicity [2%]), 44 (92%) completed the trial. Geometric mean maximum plasma concentrations of all 6 active ingredients were greater than 0.5 ng/mL, and this threshold was surpassed on day 1 after a single application for all active ingredients. For avobenzone, the overall maximum plasma concentrations were 7.1 ng/mL (coefficient of variation [CV], 73.9%) for lotion, 3.5 ng/mL (CV, 70.9%) for aerosol spray, 3.5 ng/mL (CV, 73.0%) for nonaerosol spray, and 3.3 ng/mL (CV, 47.8%) for pump spray. For oxybenzone, the concentrations were 258.1 ng/mL (CV, 53.0%) for lotion and 180.1 ng/mL (CV, 57.3%) for aerosol spray. For octocrylene, the concentrations were 7.8 ng/mL (CV, 87.1%) for lotion, 6.6 ng/mL (CV, 78.1%) for aerosol spray, and 6.6 ng/mL (CV, 103.9%) for nonaerosol spray. For homosalate, concentrations were 23.1 ng/mL (CV, 68.0%) for aerosol spray, 17.9 ng/mL (CV, 61.7%) for nonaerosol spray, and 13.9 ng/mL (CV, 70.2%) for pump spray. For octisalate, concentrations were 5.1 ng/mL (CV, 81.6%) for aerosol spray, 5.8 ng/mL (CV, 77.4%) for nonaerosol spray, and 4.6 ng/mL (CV, 97.6%) for pump spray. For octinoxate, concentrations were 7.9 ng/mL (CV, 86.5%) for nonaerosol spray and 5.2 ng/mL (CV, 68.2%) for pump spray. The most common adverse event was rash, which developed in 14 participants. Conclusions and Relevance
In this study conducted in a clinical pharmacology unit and examining sunscreen application among healthy participants, all 6 of the tested active ingredients administered in 4 different sunscreen formulations were systemically absorbed and had plasma concentrations that surpassed the FDA threshold for potentially waiving some of the additional safety studies for sunscreens. These findings do not indicate that individuals should refrain from the use of sunscreen. Trial Registration
ClinicalTrials.gov Identifier: NCT03582215.
中文翻译:

防晒霜应用对防晒霜活性成分血浆浓度的影响
重要性 一项先前的试点研究证明了 4 种防晒活性成分的全身吸收;根据美国食品和药物管理局 (FDA) 的建议,还需要更多研究来确定其他活性成分的全身吸收以及全身暴露超过 0.5 ng/mL 的速度。目的 评估 4 种防晒产品中 6 种活性成分(阿伏苯宗、羟苯甲酮、奥克立林、高水杨酸盐、辛酸和辛诺酸盐)在单次和最大使用量条件下的全身吸收和药代动力学。设计、设置和参与者 在临床药理学单位(威斯康星州西本德)对 48 名健康参与者进行了随机临床试验。该研究于 2019 年 1 月至 2 月期间进行。 干预措施 参与者被随机分配到 4 种防晒产品中的一种,配制为乳液 (n = 12)、气溶胶喷雾 (n = 12)、非气溶胶喷雾 (n = 12) 和泵喷雾 (n = 12)。防晒产品在第 1 天的 0 小时和第 2 天到第 4 天的 4 次以 2 毫克/平方厘米的剂量应用到体表面积的 75%,间隔 2 小时,并在 21 天内从每位参与者身上收集了 34 份血液样本。主要结果和措施 主要结果是第 1 天至第 21 天阿伏苯宗的最大血浆浓度。次要结果是第 1 天至第 21 天的羟苯酮、奥克立林、高水杨酸盐、辛酸和辛氧酸盐的最大血浆浓度。 结果 48 名随机参与者(平均 [SD] 年龄,38.7 [13.2] 岁;24 名女性 [50%];23 名白人 [48%],23 名非裔美国人 [48%],1 名亚洲人 [2%],以及 1 名未知种族/民族 [2 %]),44 (92%) 完成了试验。所有 6 种活性成分的几何平均最大血浆浓度均大于 0.5 ng/mL,并且在单次应用所有活性成分后的第 1 天超过了该阈值。对于阿伏苯宗,乳液的总体最大血浆浓度为 7.1 ng/mL(变异系数 [CV],73.9%),气雾剂喷雾剂为 3.5 ng/mL(CV,70.9%),3.5 ng/mL(CV,73.0%) ) 用于非气溶胶喷雾,3.3 ng/mL (CV, 47.8%) 用于泵喷雾。对于氧苯酮,乳液的浓度为 258.1 ng/mL(CV,53.0%),气雾剂喷雾的浓度为 180.1 ng/mL(CV,57.3%)。对于奥克立林,乳液的浓度为 7.8 ng/mL(CV,87.1%),气溶胶喷雾的浓度为 6.6 ng/mL(CV,78.1%),非气溶胶喷雾的浓度为 6.6 ng/mL(CV,103.9%)。对于高草酸盐,气溶胶喷雾的浓度为 23.1 ng/mL(CV,68.0%),非气溶胶喷雾的浓度为 17.9 ng/mL(CV,61.7%),和 13.9 ng/mL (CV, 70.2%) 用于泵喷雾。对于辛酸,气溶胶喷雾的浓度为 5.1 ng/mL(CV,81.6%),非气溶胶喷雾的浓度为 5.8 ng/mL(CV,77.4%),泵喷雾的浓度为 4.6 ng/mL(CV,97.6%)。对于辛酸,非气溶胶喷雾的浓度为 7.9 ng/mL(CV,86.5%),泵喷雾的浓度为 5.2 ng/mL(CV,68.2%)。最常见的不良事件是皮疹,在 14 名参与者中出现。结论和相关性 在临床药理学单位进行的这项研究中,检查了健康参与者的防晒霜应用,在 4 种不同防晒配方中施用的所有 6 种测试活性成分都被全身吸收,并且血浆浓度超过了 FDA 阈值,可能会放弃一些防晒霜的额外安全研究。这些发现并不表明个人应该避免使用防晒霜。试验注册 ClinicalTrials.gov 标识符:NCT03582215。
更新日期:2020-01-21
中文翻译:

防晒霜应用对防晒霜活性成分血浆浓度的影响
重要性 一项先前的试点研究证明了 4 种防晒活性成分的全身吸收;根据美国食品和药物管理局 (FDA) 的建议,还需要更多研究来确定其他活性成分的全身吸收以及全身暴露超过 0.5 ng/mL 的速度。目的 评估 4 种防晒产品中 6 种活性成分(阿伏苯宗、羟苯甲酮、奥克立林、高水杨酸盐、辛酸和辛诺酸盐)在单次和最大使用量条件下的全身吸收和药代动力学。设计、设置和参与者 在临床药理学单位(威斯康星州西本德)对 48 名健康参与者进行了随机临床试验。该研究于 2019 年 1 月至 2 月期间进行。 干预措施 参与者被随机分配到 4 种防晒产品中的一种,配制为乳液 (n = 12)、气溶胶喷雾 (n = 12)、非气溶胶喷雾 (n = 12) 和泵喷雾 (n = 12)。防晒产品在第 1 天的 0 小时和第 2 天到第 4 天的 4 次以 2 毫克/平方厘米的剂量应用到体表面积的 75%,间隔 2 小时,并在 21 天内从每位参与者身上收集了 34 份血液样本。主要结果和措施 主要结果是第 1 天至第 21 天阿伏苯宗的最大血浆浓度。次要结果是第 1 天至第 21 天的羟苯酮、奥克立林、高水杨酸盐、辛酸和辛氧酸盐的最大血浆浓度。 结果 48 名随机参与者(平均 [SD] 年龄,38.7 [13.2] 岁;24 名女性 [50%];23 名白人 [48%],23 名非裔美国人 [48%],1 名亚洲人 [2%],以及 1 名未知种族/民族 [2 %]),44 (92%) 完成了试验。所有 6 种活性成分的几何平均最大血浆浓度均大于 0.5 ng/mL,并且在单次应用所有活性成分后的第 1 天超过了该阈值。对于阿伏苯宗,乳液的总体最大血浆浓度为 7.1 ng/mL(变异系数 [CV],73.9%),气雾剂喷雾剂为 3.5 ng/mL(CV,70.9%),3.5 ng/mL(CV,73.0%) ) 用于非气溶胶喷雾,3.3 ng/mL (CV, 47.8%) 用于泵喷雾。对于氧苯酮,乳液的浓度为 258.1 ng/mL(CV,53.0%),气雾剂喷雾的浓度为 180.1 ng/mL(CV,57.3%)。对于奥克立林,乳液的浓度为 7.8 ng/mL(CV,87.1%),气溶胶喷雾的浓度为 6.6 ng/mL(CV,78.1%),非气溶胶喷雾的浓度为 6.6 ng/mL(CV,103.9%)。对于高草酸盐,气溶胶喷雾的浓度为 23.1 ng/mL(CV,68.0%),非气溶胶喷雾的浓度为 17.9 ng/mL(CV,61.7%),和 13.9 ng/mL (CV, 70.2%) 用于泵喷雾。对于辛酸,气溶胶喷雾的浓度为 5.1 ng/mL(CV,81.6%),非气溶胶喷雾的浓度为 5.8 ng/mL(CV,77.4%),泵喷雾的浓度为 4.6 ng/mL(CV,97.6%)。对于辛酸,非气溶胶喷雾的浓度为 7.9 ng/mL(CV,86.5%),泵喷雾的浓度为 5.2 ng/mL(CV,68.2%)。最常见的不良事件是皮疹,在 14 名参与者中出现。结论和相关性 在临床药理学单位进行的这项研究中,检查了健康参与者的防晒霜应用,在 4 种不同防晒配方中施用的所有 6 种测试活性成分都被全身吸收,并且血浆浓度超过了 FDA 阈值,可能会放弃一些防晒霜的额外安全研究。这些发现并不表明个人应该避免使用防晒霜。试验注册 ClinicalTrials.gov 标识符:NCT03582215。











































 京公网安备 11010802027423号
京公网安备 11010802027423号