Environmental Pollution ( IF 7.6 ) Pub Date : 2020-01-20 , DOI: 10.1016/j.envpol.2020.114019 Sunday J Olusegun 1 , Nelcy D S Mohallem 1
|
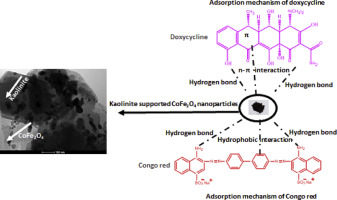
Kaolinite supported CoFe2O4 (KCF) was synthesized and employed to adsorb doxycycline (DOX), an antibiotic and Congo red (CR), a dye from aqueous solution. The prepared KCF nanocomposite was treated in a muffle furnace at 300, 500 and 700 °C, and thereafter characterized. X-ray diffractogram revealed structural damage of kaolinite and appearance of distinct peaks of CoFe2O4 with an increase in calcination temperature, while transmission electron microscopy (TEM) images showed that CoFe2O4 nanoparticles were supported on the lamellar surface of kaolinites. Comparative adsorption mechanism of the two targeted contaminants showed that adsorption of DOX was influenced by hydrogen bond and n-π interaction, while that of CR was due to hydrophobic interaction and hydrogen bond. However, the adsorption of the two contaminants was best fitted to the isotherm that was proposed by Langmuir, with a monolayer maximum adsorption capacity of 400 mg g−1 at 333 K for DOX, and 547 mg g−1 at 298 K for CR. The removal of DOX from aqueous solution was favored by an increase in temperature (endothermic), while that of CR was exothermic. Thermodynamics studies confirmed that the adsorption of the two contaminants is feasible and spontaneous. The presence of natural organic matter (NOM) did not affect the removal of the two contaminants. Regeneration and reusability study showed that KCF is economically viable. Therefore, introducing inorganic particles like cobalt ferrite into the matrix of kaolinites provides a composite with promising adsorption capacity.
中文翻译:

使用合成的高岭石负载的CoFe2O4纳米颗粒比较强力霉素和刚果红的吸附机理。
合成了高岭石负载的CoFe 2 O 4(KCF),并用于从水溶液中吸附强力霉素(DOX)(一种抗生素)和刚果红(CR)(一种染料)。将制得的KCF纳米复合材料在马弗炉中于300、500和700℃下处理,然后进行表征。X射线衍射图显示,随着煅烧温度的升高,高岭石的结构破坏和CoFe 2 O 4的明显峰的出现,而透射电子显微镜(TEM)图像显示CoFe 2 O 4纳米颗粒被支撑在高岭石的层状表面上。两种目标污染物的比较吸附机理表明,DOX的吸附受氢键和n-π相互作用的影响,而CR的吸附则受疏水作用和氢键的影响。但是,两种污染物的吸附最适合Langmuir提出的等温线,其在333 K下对DOX的单层最大吸附容量为400 mg g -1,对于547 mg g -1CR为298K。温度升高(吸热)有利于从水溶液中除去DOX,而CR则放热。热力学研究证实,两种污染物的吸附是可行且自发的。天然有机物(NOM)的存在不影响两种污染物的去除。再生和可重用性研究表明,KCF具有经济可行性。因此,将诸如钴铁氧体的无机颗粒引入到高岭石的基质中,提供了具有希望的吸附能力的复合材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号