当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Re-assembled oleic acid-protein complexes as nano-vehicles for astaxanthin: multispectral analysis and molecular docking
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.foodhyd.2020.105689 Yixiang Liu , Ling Huang , Donghui Li , Yanbo Wang , Zhaohua Chen , Chao Zou , Wenqiang Liu , Yu Ma , Min-Jie Cao , Guang-Ming Liu
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.foodhyd.2020.105689 Yixiang Liu , Ling Huang , Donghui Li , Yanbo Wang , Zhaohua Chen , Chao Zou , Wenqiang Liu , Yu Ma , Min-Jie Cao , Guang-Ming Liu
|
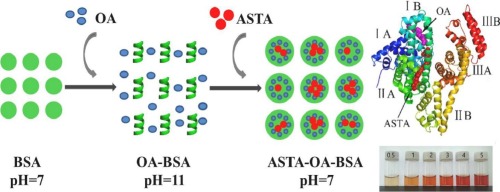
Abstract In the case of carotenoids, lipid delivery systems are superior in promoting intestinal absorption, while biopolymer-based delivery systems (e.g. protein) have advantages in biocompatibility, biodegradability and controlled release. Therefore, based on the multi-ligand binding properties of protein, we proposed fatty acid-protein complexes as nano-vehicles for encapsulating astaxanthin (ASTA). In this work, oleic acid (OA)–bovine serum albumin (BSA) complexes were fabricated by a pH-driven method. Under the optimized conditions, after OA binding to BSA at a molar ratio of 4:1, slight changes of protein secondary structure appeared and it's surface hydrophobicity increased from 14490 to 25324. When the binding molar ratios of ASTA to BSA ranged from 0.5:1 to 5:1, the ASTA–OA–BSA aqueous dispersions showed better clarity than that of ASTA–BSA. Compared with OA-free BSA, improved encapsulation efficiency and loading capacity were also observed for OA–loading BSA. Based on the fluorescence spectra and Stern–Volmer equation analysis, ASTA binding to the OA-loading BSA displayed a static manner mainly through hydrogen binding and van der Waals interactions. According to the results of site marker competitive experiments and molecular docking, the subdomains IIA and IIIA of BSA should be the acceptor sites of ASTA, and the subdomain IIIA was indicated to be the preferential one. Interestingly, OA as a protein ligand can dramatically enhanced the binding capacity of subdomain IIA to ASTA and make it the preferred acceptor site. In conclusion, OA–BSA nanoparticles as a delivery system should be suitable for fat-soluble nutrients such as carotenoids.
中文翻译:

重组油酸-蛋白质复合物作为虾青素纳米载体:多光谱分析和分子对接
摘要 以类胡萝卜素为例,脂质递送系统在促进肠道吸收方面具有优势,而基于生物聚合物的递送系统(例如蛋白质)在生物相容性、生物降解性和控释方面具有优势。因此,基于蛋白质的多配体结合特性,我们提出了脂肪酸-蛋白质复合物作为封装虾青素(ASTA)的纳米载体。在这项工作中,油酸 (OA)-牛血清白蛋白 (BSA) 复合物是通过 pH 驱动的方法制备的。在优化条件下,OA与BSA以4:1的摩尔比结合后,蛋白质二级结构发生轻微变化,表面疏水性从14490增加到25324。当ASTA与BSA的结合摩尔比为0.5:1时到 5:1, ASTA-OA-BSA 水分散体比 ASTA-BSA 水分散体表现出更好的透明度。与不含 OA 的 BSA 相比,加载 OA 的 BSA 的包封效率和负载能力也有所提高。基于荧光光谱和 Stern-Volmer 方程分析,ASTA 与负载 OA 的 BSA 的结合主要通过氢结合和范德华相互作用显示出静态方式。根据位点标记竞争实验和分子对接的结果,BSA的亚域IIA和IIIA应该是ASTA的受体位点,亚域IIIA被认为是优先位点。有趣的是,OA 作为蛋白质配体可以显着增强子域 IIA 与 ASTA 的结合能力,使其成为首选的受体位点。综上所述,
更新日期:2020-06-01
中文翻译:

重组油酸-蛋白质复合物作为虾青素纳米载体:多光谱分析和分子对接
摘要 以类胡萝卜素为例,脂质递送系统在促进肠道吸收方面具有优势,而基于生物聚合物的递送系统(例如蛋白质)在生物相容性、生物降解性和控释方面具有优势。因此,基于蛋白质的多配体结合特性,我们提出了脂肪酸-蛋白质复合物作为封装虾青素(ASTA)的纳米载体。在这项工作中,油酸 (OA)-牛血清白蛋白 (BSA) 复合物是通过 pH 驱动的方法制备的。在优化条件下,OA与BSA以4:1的摩尔比结合后,蛋白质二级结构发生轻微变化,表面疏水性从14490增加到25324。当ASTA与BSA的结合摩尔比为0.5:1时到 5:1, ASTA-OA-BSA 水分散体比 ASTA-BSA 水分散体表现出更好的透明度。与不含 OA 的 BSA 相比,加载 OA 的 BSA 的包封效率和负载能力也有所提高。基于荧光光谱和 Stern-Volmer 方程分析,ASTA 与负载 OA 的 BSA 的结合主要通过氢结合和范德华相互作用显示出静态方式。根据位点标记竞争实验和分子对接的结果,BSA的亚域IIA和IIIA应该是ASTA的受体位点,亚域IIIA被认为是优先位点。有趣的是,OA 作为蛋白质配体可以显着增强子域 IIA 与 ASTA 的结合能力,使其成为首选的受体位点。综上所述,











































 京公网安备 11010802027423号
京公网安备 11010802027423号