当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The radiation assisted-Maillard reaction comprehensively improves the freeze-thaw stability of soy protein-stabilized oil-in-water emulsions
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.foodhyd.2020.105684 Yuying Wang , Anqi Zhang , Xibo Wang , Ning Xu , Lianzhou Jiang
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.foodhyd.2020.105684 Yuying Wang , Anqi Zhang , Xibo Wang , Ning Xu , Lianzhou Jiang
|
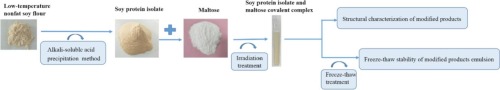
Abstract For the first time, the irradiation technology combined with Maillard reaction were used to improve the freeze - thaw stability of soybean protein emulsion. The freeze-thaw stability of emulsions prepared with soy protein isolate (SPI), soy protein isolate and maltose mixture (SPI + M) or glycosylated soy protein isolate with maltose formed by irradiation of 7.5 kGy and 12.5 kGy (named SPI-M7.5 and SPI-M12.5, respectively) was compared. Fourier transform infrared spectroscopy, fluorescence spectroscopy and ultraviolet spectroscopy confirmed the change of the structure of soy protein isolate, indicating that maltose was covalently linked to soybean protein isolate. Scanning electron microscopy showed that the modified protein particles were more loose, uniform in size, and significantly reduced in molecular aggregation than untreated protein. The freeze-thaw stability of SPI, SPI + M, and SPI-M7.5 and SPI-M12.5 emulsions was evaluated. It was found that after three freeze-thaw cycles, the creaming index, oiling off, particle size, coalescence degree and flocculation degree of emulsions prepared with irradiated SPI samples were all lower than those prepared with SPI and SPI + M. Zeta potential, laser confocal and optical microscopy showed that the emulsion was still in a relatively stable state, and the SPI-M7.5 emulsion after freeze-thaw treatment was more stable than the SPI-M12.5 emulsion.
中文翻译:

辐射辅助美拉德反应全面提高大豆蛋白稳定水包油乳液的冻融稳定性
摘要 首次采用辐照技术结合美拉德反应提高大豆蛋白乳剂的冻融稳定性。用大豆分离蛋白 (SPI)、大豆分离蛋白和麦芽糖混合物 (SPI + M) 或经 7.5 kGy 和 12.5 kGy 辐照形成的麦芽糖糖基化大豆分离蛋白制备的乳液的冻融稳定性(命名为 SPI-M7.5和 SPI-M12.5)进行了比较。傅里叶变换红外光谱、荧光光谱和紫外光谱证实了大豆分离蛋白结构的变化,表明麦芽糖与大豆分离蛋白共价连接。扫描电镜显示,修饰后的蛋白质颗粒更松散,大小均匀,与未处理的蛋白质相比,分子聚集显着减少。评估了 SPI、SPI + M 和 SPI-M7.5 和 SPI-M12.5 乳液的冻融稳定性。发现经3次冻融循环后,经辐照的SPI样品制备的乳液的乳化指数、脱油率、粒径、聚结度和絮凝度均低于SPI和SPI+M。 Zeta电位、激光共聚焦和光学显微镜显示乳液仍处于相对稳定的状态,冻融处理后的SPI-M7.5乳液比SPI-M12.5乳液更稳定。
更新日期:2020-06-01
中文翻译:

辐射辅助美拉德反应全面提高大豆蛋白稳定水包油乳液的冻融稳定性
摘要 首次采用辐照技术结合美拉德反应提高大豆蛋白乳剂的冻融稳定性。用大豆分离蛋白 (SPI)、大豆分离蛋白和麦芽糖混合物 (SPI + M) 或经 7.5 kGy 和 12.5 kGy 辐照形成的麦芽糖糖基化大豆分离蛋白制备的乳液的冻融稳定性(命名为 SPI-M7.5和 SPI-M12.5)进行了比较。傅里叶变换红外光谱、荧光光谱和紫外光谱证实了大豆分离蛋白结构的变化,表明麦芽糖与大豆分离蛋白共价连接。扫描电镜显示,修饰后的蛋白质颗粒更松散,大小均匀,与未处理的蛋白质相比,分子聚集显着减少。评估了 SPI、SPI + M 和 SPI-M7.5 和 SPI-M12.5 乳液的冻融稳定性。发现经3次冻融循环后,经辐照的SPI样品制备的乳液的乳化指数、脱油率、粒径、聚结度和絮凝度均低于SPI和SPI+M。 Zeta电位、激光共聚焦和光学显微镜显示乳液仍处于相对稳定的状态,冻融处理后的SPI-M7.5乳液比SPI-M12.5乳液更稳定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号