Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study of the noncovalent interactions between phenolic acid and lysozyme by cold spray ionization mass spectrometry (CSI-MS), multi-spectroscopic and molecular docking approaches.
Talanta ( IF 5.6 ) Pub Date : 2020-01-18 , DOI: 10.1016/j.talanta.2020.120762 Su Chen 1 , Xin Gong 2 , Hongwei Tan 2 , Yang Liu 3 , Lan He 3 , Jin Ouyang 2
Talanta ( IF 5.6 ) Pub Date : 2020-01-18 , DOI: 10.1016/j.talanta.2020.120762 Su Chen 1 , Xin Gong 2 , Hongwei Tan 2 , Yang Liu 3 , Lan He 3 , Jin Ouyang 2
Affiliation
|
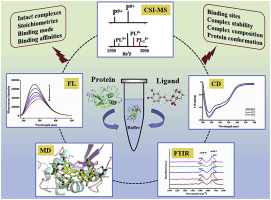
Elucidating the recognition mechanisms of the noncovalent interactions between pharmaceutical molecules and proteins is important for understanding drug delivery in vivo, and for the further rapid screening of clinical drug candidates and biomarkers. In this work, a strategy based on cold spray ionization mass spectrometry (CSI-MS), combined with fluorescence, circular dichroism (CD), Fourier transform infrared spectroscopy (FTIR), and molecular docking methods, was developed and applied to the study of the noncovalent interactions between phenolic acid and lysozyme (Lys). Based on the real characterization of noncovalent complex, the detailed binding parameters, as well as the protein conformational changes and specific binding sites could be obtained. CSI-MS and tandem mass spectrometry (MS/MS) technique were used to investigate the phenolic acid-Lys complexes and the structure-affinity relationship, and to assess their structural composition and gas phase stability. The binding affinity was obtained by direct and indirect MS methods. The fluorescence spectra showed that the intrinsic fluorescence quenching of Lys in solution was a static quenching mechanism caused by complex formation, which supported the MS results. The CD and FTIR spectra revealed that phenolic acid changed the secondary structure of Lys and increased the α-helix content, indicating an increase in the tryptophan (W) hydrophobicity near the protein binding site resulting in a conformational alteration of the protein. In addition, molecular docking studies were performed to investigate the binding sites and binding modes of phenolic acid on Lys. This strategy can more comprehensively and truly characterize the noncovalent interactions and can guide further research on the interactions of phenolic acid with other proteins.
中文翻译:

通过冷喷雾电离质谱(CSI-MS),多光谱和分子对接方法研究酚酸和溶菌酶之间的非共价相互作用。
阐明药物分子与蛋白质之间非共价相互作用的识别机制对于理解体内药物传递以及进一步快速筛选临床候选药物和生物标记物非常重要。在这项工作中,开发了一种基于冷喷雾电离质谱(CSI-MS)并结合荧光,圆二色性(CD),傅立叶变换红外光谱(FTIR)和分子对接方法的策略,并将其应用于研究酚酸和溶菌酶(Lys)之间的非共价相互作用。基于非共价复合物的真实表征,可以获得详细的结合参数,以及蛋白质构象变化和特异性结合位点。利用CSI-MS和串联质谱(MS / MS)技术研究了酚酸-Lys配合物及其结构亲和性,并评估了其结构组成和气相稳定性。通过直接和间接MS方法获得结合亲和力。荧光光谱表明,溶液中Lys的固有荧光猝灭是复合物形成引起的静态猝灭机制,这支持了MS结果。CD和FTIR光谱表明,酚酸改变了Lys的二级结构并增加了α-螺旋含量,表明在蛋白质结合位点附近的色氨酸(W)疏水性增加,从而导致蛋白质的构象改变。此外,进行了分子对接研究以研究酚酸在Lys上的结合位点和结合方式。该策略可以更全面,更真实地表征非共价相互作用,并且可以指导进一步研究酚酸与其他蛋白质的相互作用。
更新日期:2020-01-21
中文翻译:

通过冷喷雾电离质谱(CSI-MS),多光谱和分子对接方法研究酚酸和溶菌酶之间的非共价相互作用。
阐明药物分子与蛋白质之间非共价相互作用的识别机制对于理解体内药物传递以及进一步快速筛选临床候选药物和生物标记物非常重要。在这项工作中,开发了一种基于冷喷雾电离质谱(CSI-MS)并结合荧光,圆二色性(CD),傅立叶变换红外光谱(FTIR)和分子对接方法的策略,并将其应用于研究酚酸和溶菌酶(Lys)之间的非共价相互作用。基于非共价复合物的真实表征,可以获得详细的结合参数,以及蛋白质构象变化和特异性结合位点。利用CSI-MS和串联质谱(MS / MS)技术研究了酚酸-Lys配合物及其结构亲和性,并评估了其结构组成和气相稳定性。通过直接和间接MS方法获得结合亲和力。荧光光谱表明,溶液中Lys的固有荧光猝灭是复合物形成引起的静态猝灭机制,这支持了MS结果。CD和FTIR光谱表明,酚酸改变了Lys的二级结构并增加了α-螺旋含量,表明在蛋白质结合位点附近的色氨酸(W)疏水性增加,从而导致蛋白质的构象改变。此外,进行了分子对接研究以研究酚酸在Lys上的结合位点和结合方式。该策略可以更全面,更真实地表征非共价相互作用,并且可以指导进一步研究酚酸与其他蛋白质的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号