当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expression and Purification of Protease-Activated Receptor 4 (PAR4) and Analysis with Histidine Hydrogen-Deuterium Exchange.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-20 , DOI: 10.1021/acs.biochem.9b00987 Maria de la Fuente 1 , Xu Han 1 , Masaru Miyagi 1 , Marvin T Nieman 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-20 , DOI: 10.1021/acs.biochem.9b00987 Maria de la Fuente 1 , Xu Han 1 , Masaru Miyagi 1 , Marvin T Nieman 1
Affiliation
|
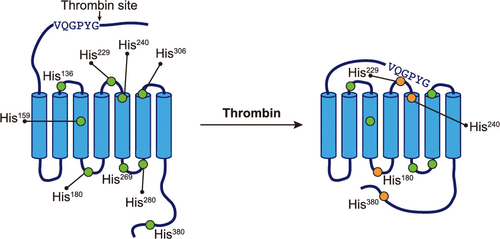
Protease-activated receptors (PARs) are G-protein-coupled receptors that are activated by proteolysis of the N-terminus, which exposes a tethered ligand that interacts with the receptor. Numerous studies have focused on the signaling pathways mediated by PARs. However, the structural basis for initiation of these pathways is unknown. Here, we describe a strategy for the expression and purification of PAR4. This is the first PAR family member to be isolated without stabilizing modifications for biophysical studies. We monitored PAR4 activation with histidine hydrogen-deuterium exchange. PAR4 has nine histidines that are spaced throughout the protein, allowing a global view of solvent accessible and nonaccessible regions. Peptides containing each of the nine His residues were used to determine the t1/2 for each His residue in apo or thrombin-activated PAR4. The thrombin-cleaved PAR4 exhibited a 2-fold increase (p > 0.01) in t1/2 values observed for four histidine residues (His180, His229, His240, and His380), demonstrating that these regions have decreased solvent accessibility upon thrombin treatment. In agreement, thrombin-cleaved PAR4 also was resistant to thermolysin digestion. In contrast, the rate of thermolysin proteolysis following stimulation with the PAR4 activation peptide was the same as that of unstimulated PAR4. Further analysis showed the C-terminus is protected in thrombin-activated PAR4 compared to uncleaved or agonist peptide-treated PAR4. The studies described here are the first to examine the tethered ligand activation mechanism for a PAR family member biophysically and shed light on the overall conformational changes that follow activation of PARs by a protease.
中文翻译:

蛋白酶激活受体 4 (PAR4) 的表达和纯化以及组氨酸氢-氘交换分析。
蛋白酶激活受体 (PAR) 是 G 蛋白偶联受体,可通过 N 末端的蛋白水解激活,从而暴露出与受体相互作用的束缚配体。许多研究都集中在 PAR 介导的信号通路上。然而,启动这些途径的结构基础是未知的。在这里,我们描述了 PAR4 的表达和纯化策略。这是第一个在没有为生物物理研究进行稳定修饰的情况下被分离出来的 PAR 家族成员。我们通过组氨酸氢-氘交换监测 PAR4 激活。PAR4 有九个组氨酸,它们分布在整个蛋白质中,从而可以全局查看溶剂可及和不可及的区域。含有九个 His 残基中的每一个的肽用于确定 apo 或凝血酶激活的 PAR4 中每个 His 残基的 t1/2。凝血酶切割的 PAR4 在观察到的四个组氨酸残基(His180、His229、His240 和 His380)的 t1/2 值中表现出 2 倍的增加 (p > 0.01),表明这些区域在凝血酶处理后降低了溶剂可及性。同意的是,凝血酶切割的 PAR4 也对嗜热菌蛋白酶消化具有抗性。相反,用 PAR4 激活肽刺激后的嗜热菌蛋白酶蛋白水解速率与未刺激的 PAR4 相同。进一步分析表明,与未切割或激动剂肽处理的 PAR4 相比,C 末端在凝血酶激活的 PAR4 中受到保护。
更新日期:2020-01-24
中文翻译:

蛋白酶激活受体 4 (PAR4) 的表达和纯化以及组氨酸氢-氘交换分析。
蛋白酶激活受体 (PAR) 是 G 蛋白偶联受体,可通过 N 末端的蛋白水解激活,从而暴露出与受体相互作用的束缚配体。许多研究都集中在 PAR 介导的信号通路上。然而,启动这些途径的结构基础是未知的。在这里,我们描述了 PAR4 的表达和纯化策略。这是第一个在没有为生物物理研究进行稳定修饰的情况下被分离出来的 PAR 家族成员。我们通过组氨酸氢-氘交换监测 PAR4 激活。PAR4 有九个组氨酸,它们分布在整个蛋白质中,从而可以全局查看溶剂可及和不可及的区域。含有九个 His 残基中的每一个的肽用于确定 apo 或凝血酶激活的 PAR4 中每个 His 残基的 t1/2。凝血酶切割的 PAR4 在观察到的四个组氨酸残基(His180、His229、His240 和 His380)的 t1/2 值中表现出 2 倍的增加 (p > 0.01),表明这些区域在凝血酶处理后降低了溶剂可及性。同意的是,凝血酶切割的 PAR4 也对嗜热菌蛋白酶消化具有抗性。相反,用 PAR4 激活肽刺激后的嗜热菌蛋白酶蛋白水解速率与未刺激的 PAR4 相同。进一步分析表明,与未切割或激动剂肽处理的 PAR4 相比,C 末端在凝血酶激活的 PAR4 中受到保护。









































 京公网安备 11010802027423号
京公网安备 11010802027423号